InVivoMAb anti-mouse IL-21R
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Rat YB2/0 cell line expressing truncated IL-21 R |
| Reported Applications | in vivo IL-21R blockade |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687737 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo IL-21R blockade
Clemente-Casares, X., et al (2016). "Expanding antigen-specific regulatory networks to treat autoimmunity" Nature 530(7591): 434-440.
PubMed
Regulatory T cells hold promise as targets for therapeutic intervention in autoimmunity, but approaches capable of expanding antigen-specific regulatory T cells in vivo are currently not available. Here we show that systemic delivery of nanoparticles coated with autoimmune-disease-relevant peptides bound to major histocompatibility complex class II (pMHCII) molecules triggers the generation and expansion of antigen-specific regulatory CD4(+) T cell type 1 (TR1)-like cells in different mouse models, including mice humanized with lymphocytes from patients, leading to resolution of established autoimmune phenomena. Ten pMHCII-based nanomedicines show similar biological effects, regardless of genetic background, prevalence of the cognate T-cell population or MHC restriction. These nanomedicines promote the differentiation of disease-primed autoreactive T cells into TR1-like cells, which in turn suppress autoantigen-loaded antigen-presenting cells and drive the differentiation of cognate B cells into disease-suppressing regulatory B cells, without compromising systemic immunity. pMHCII-based nanomedicines thus represent a new class of drugs, potentially useful for treating a broad spectrum of autoimmune conditions in a disease-specific manner.
in vivo IL-21R blockade
Cao, A. T., et al (2015). "Interleukin (IL)-21 promotes intestinal IgA response to microbiota" Mucosal Immunol 8(5): 1072-1082.
PubMed
Commensal microbiota-specific T helper type 17 (Th17) cells are enriched in the intestines, which can convert into T follicular helper (Tfh) in Peyer’s patches, and are crucial for production of intestinal immunoglobulin A (IgA) against microbiota; however, the role of Th17 and Tfh cytokines in regulating the mucosal IgA response to enteric microbiota is still not completely known. In this study, we found that intestinal IgA was impaired in mice deficient in interleukin (IL)-17 or IL-21 signaling. IL-21, but not IL-17, is able to augment B-cell differentiation to IgA(+) cells as mediated by transforming growth factor beta1 (TGFbeta1) and accelerate IgA class switch recombination (CSR). IL-21 and retinoic acid (RA) induce IgA(+) B-cell development and IgA production and drives autocrine TGFbeta1 production to initiate IgA CSR. Repletion of T-cell-deficient TCRbetaxdelta(-/-) mice with Th17 cells specific for commensal bacterial antigen increased the levels of IgA(+) B cells and IgA production in the intestine, which was blocked by neutralizing IL-21. Thus IL-21 functions to strongly augment IgA production under intestinal environment. Furthermore, IL-21 promotes intestinal B-cell homing through alpha4beta7 expression, alone or with TGFbeta and RA. Together, IL-21 from microbiota-specific Th17 and/or Tfh cells contributes to robust intestinal IgA levels by enhancing IgA(+) CSR, IgA production and B-cell trafficking into the intestine.
Product Citations
-
-
Cell Biology
-
Immunology and Microbiology
Probiotics and their metabolite spermidine enhance IFN-γ+CD4+ T cell immunity to inhibit hepatitis B virus.
In Cell Rep Med on 19 November 2024 by Wang, T., Fan, Y., et al.
PubMed
The therapeutic potential of commensal microbes and their metabolites is promising in the functional cure of chronic hepatitis B virus (HBV) infection, which is defined as hepatitis B surface antigen (HBsAg) loss. Here, using both specific-pathogen-free and germ-free mice, we report that probiotics significantly promote the decline of HBsAg and inhibit HBV replication by enhancing intestinal homeostasis and provoking intrahepatic interferon (IFN)-γ+CD4+ T cell immune response. Depletion of CD4+ T cells or blockage of IFN-γ abolishes probiotics-mediated HBV inhibition. Specifically, probiotics-derived spermidine accumulates in the gut and transports to the liver, where it exhibits a similar anti-HBV effect. Mechanistically, spermidine enhances IFN-γ+CD4+ T cell immunity by autophagy. Strikingly, administration of probiotics in HBV patients reveals a preliminary trend to accelerate the decline of serum HBsAg. In conclusion, probiotics and their derived spermidine promote HBV clearance via autophagy-enhanced IFN-γ+CD4+ T cell immunity, highlighting the therapeutic potential of probiotics and spermidine for the functional cure of HBV patients.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
A pathologically expanded, clonal lineage of IL-21-producing CD4+ T cells drives inflammatory neuropathy.
In J Clin Invest on 11 June 2024 by Seyedsadr, M., Bang, M. F., et al.
PubMed
Inflammatory neuropathies, which include chronic inflammatory demyelinating polyneuropathy (CIDP) and Guillain Barré syndrome (GBS), result from autoimmune destruction of the PNS and are characterized by progressive weakness and sensory loss. CD4+ T cells play a key role in the autoimmune destruction of the PNS. Yet, key properties of pathogenic CD4+ T cells remain incompletely understood. Here, we used paired single-cell RNA-Seq (scRNA-Seq) and single-cell T cell receptor-sequencing (scTCR-Seq) of peripheral nerves from an inflammatory neuropathy mouse model to identify IL-21-expressing CD4+ T cells that were clonally expanded and multifunctional. These IL-21-expressing CD4+ T cells consisted of 2 transcriptionally distinct expanded cell populations, which expressed genes associated with T follicular helper (Tfh) and T peripheral helper (Tph) cell subsets. Remarkably, TCR clonotypes were shared between these 2 IL-21-expressing cell populations, suggesting a common lineage differentiation pathway. Finally, we demonstrated that IL-21 receptor-KO (IL-21R-KO) mice were protected from neuropathy development and had decreased immune infiltration into peripheral nerves. IL-21 signaling upregulated CXCR6, a chemokine receptor that promotes CD4+ T cell localization in peripheral nerves. Together, these findings point to IL-21 signaling, Tfh/Tph differentiation, and CXCR6-mediated cellular localization as potential therapeutic targets in inflammatory neuropathies.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
-
-
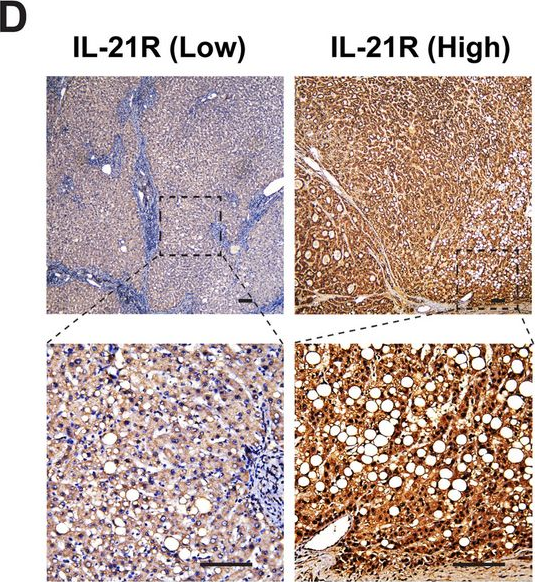
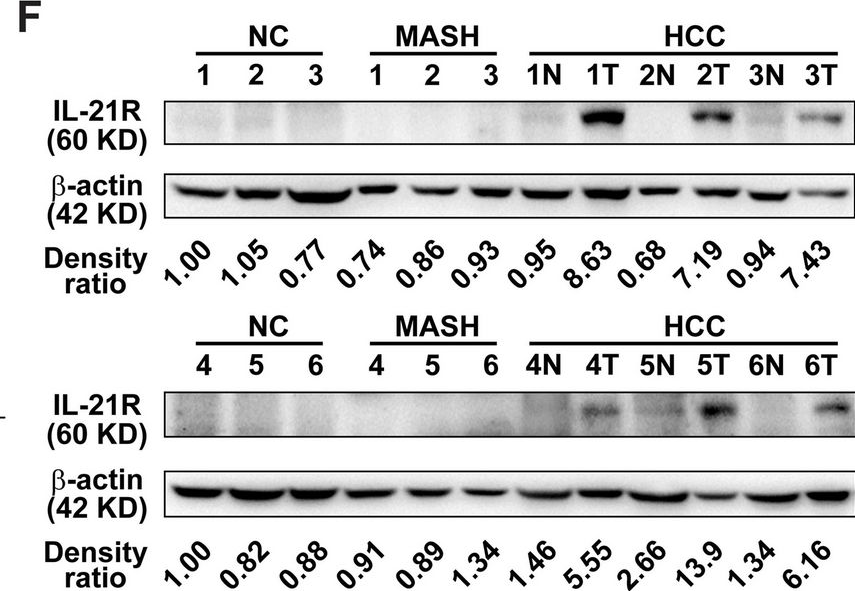
Interleukin-21 receptor signaling promotes metabolic dysfunction-associated steatohepatitis-driven hepatocellular carcinoma by inducing immunosuppressive IgA+ B cells.
In Mol Cancer on 8 May 2024 by Xie, Y., Huang, Y., et al.
PubMed
Dysregulation of immune surveillance is tightly linked to the development of metabolic dysfunction-associated steatohepatitis (MASH)-driven hepatocellular carcinoma (HCC); however, its underlying mechanisms remain unclear. Herein, we aimed to determine the role of interleukin-21 receptor (IL-21R) in MASH-driven HCC.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
An immune cell map of human lung adenocarcinoma development reveals an anti-tumoral role of the Tfh-dependent tertiary lymphoid structure.
In Cell Rep Med on 19 March 2024 by Liu, W., You, W., et al.
PubMed
The immune responses during the initiation and invasion stages of human lung adenocarcinoma (LUAD) development are largely unknown. Here, we generated a single-cell RNA sequencing map to decipher the immune dynamics during human LUAD development. We found that T follicular helper (Tfh)-like cells, germinal center B cells, and dysfunctional CD8+ T cells increase during tumor initiation/invasion and form a tertiary lymphoid structure (TLS) inside the tumor. This TLS starts with an aggregation of CD4+ T cells and the generation of CXCL13-expressing Tfh-like cells, followed by an accumulation of B cells, and then forms a CD4+ T and B cell aggregate. TLS and its associated cells are correlated with better patient survival. Inhibiting TLS formation by Tfh or B cell depletion promotes tumor growth in mouse models. The anti-tumoral effect of the Tfh-dependent TLS is mediated through interleukin-21 (IL-21)-IL-21 receptor signaling. Our study establishes an anti-tumoral role of the Tfh-dependent TLS in the development of LUAD.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Synergism Between IL21 and Anti-PD-1 Combination Therapy is Underpinned by the Coordinated Reprogramming of the Immune Cellular Network in the Tumor Microenvironment.
In Cancer Res Commun on 1 August 2023 by Wu, S., Huang, H., et al.
PubMed
T cell-stimulating cytokines and immune checkpoint inhibitors (ICI) are an ideal combination for increasing response rates of cancer immunotherapy. However, the results of clinical trials have not been satisfying. It is important to understand the mechanism of synergy between these two therapeutic modalities. Here, through integrated analysis of multiple single-cell RNA sequencing (scRNA-seq) datasets of human tumor-infiltrating immune cells, we demonstrate that IL21 is produced by tumor-associated T follicular helper cells and hyperactivated/exhausted CXCL13+CD4+ T cells in the human tumor microenvironment (TME). In the mouse model, the hyperactivated/exhausted CD4+ T cell-derived IL21 enhances the helper function of CD4+ T cells that boost CD8+ T cell-mediated immune responses during PD-1 blockade immunotherapy. In addition, we demonstrated that IL21's antitumor activity did not require T-cell trafficking. Using scRNA-seq analysis of the whole tumor-infiltrating immune cells, we demonstrated that IL21 treatment in combination with anti-PD-1 blockade synergistically drives tumor antigen-specific CD8+ T cells to undergo clonal expansion and differentiate toward the hyperactive/exhausted functional state in the TME. In addition, IL21 treatment and anti-PD-1 blockade synergistically promote dendritic cell (DC) activation and maturation to mature DC as well as monocyte to type 1 macrophage (M1) differentiation in the TME. Furthermore, the combined treatment reprograms the immune cellular network by reshaping cell-cell communication in the TME. Our study establishes unique mechanisms of synergy between IL21 and PD-1-based ICI in the TME through the coordinated promotion of type 1 immune responses.
-
-
-
Cancer Research
-
Immunology and Microbiology
STAT3 gain-of-function mutations connect leukemia with autoimmune disease by pathological NKG2Dhi CD8+ T cell dysregulation and accumulation.
In Immunity on 13 December 2022 by Masle-Farquhar, E., Jackson, K. J. L., et al.
PubMed
The association between cancer and autoimmune disease is unexplained, exemplified by T cell large granular lymphocytic leukemia (T-LGL) where gain-of-function (GOF) somatic STAT3 mutations correlate with co-existing autoimmunity. To investigate whether these mutations are the cause or consequence of CD8+ T cell clonal expansions and autoimmunity, we analyzed patients and mice with germline STAT3 GOF mutations. STAT3 GOF mutations drove the accumulation of effector CD8+ T cell clones highly expressing NKG2D, the receptor for stress-induced MHC-class-I-related molecules. This subset also expressed genes for granzymes, perforin, interferon-γ, and Ccl5/Rantes and required NKG2D and the IL-15/IL-2 receptor IL2RB for maximal accumulation. Leukocyte-restricted STAT3 GOF was sufficient and CD8+ T cells were essential for lethal pathology in mice. These results demonstrate that STAT3 GOF mutations cause effector CD8+ T cell oligoclonal accumulation and that these rogue cells contribute to autoimmune pathology, supporting the hypothesis that somatic mutations in leukemia/lymphoma driver genes contribute to autoimmune disease.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Adoptive cell therapy with tumor-specific Th9 cells induces viral mimicry to eliminate antigen-loss-variant tumor cells.
In Cancer Cell on 13 December 2021 by Xue, G., Zheng, N., et al.
PubMed
Resistance can occur in patients receiving adoptive cell therapy (ACT) due to antigen-loss-variant (ALV) cancer cell outgrowth. Here we demonstrate that murine and human T helper (Th) 9 cells, but not Th1/Tc1 or Th17 cells, expressing tumor-specific T cell receptors (TCRs) or chimeric antigen receptors (CARs), eradicate advanced tumors that contain ALVs. This unprecedented antitumor capacity of Th9 cells is attributed to both enhanced direct tumor cell killing and bystander antitumor effects promoted by intratumor release of interferon (IFN) α/β. Mechanistically, tumor-specific Th9 cells increase the intratumor accumulation of extracellular ATP (eATP; released from dying tumor cells), because of a unique feature of Th9 cells that lack the expression of ATP degrading ectoenzyme cluster of differentiation (CD) 39. Intratumor enrichment of eATP promotes the monocyte infiltration and stimulates their production of IFNα/β by inducing eATP-endogenous retrovirus-Toll-like receptor 3 (TLR3)/mitochondrial antiviral signaling (MAVS) pathway activation. These results identify tumor-specific Th9 cells as a unique T cell subset endowed with the unprecedented capacity to eliminate ALVs for curative responses.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Liver-specific T regulatory type-1 cells program local neutrophils to suppress hepatic autoimmunity via CRAMP.
In Cell Rep on 30 March 2021 by Umeshappa, C. S., Solé, P., et al.
PubMed
Neutrophils with immunoregulatory properties, also referred to as type-2 neutrophils (N2), myeloid-derived suppressor cells (MDSCs), or tumor-associated neutrophils (TANs), comprise a heterogeneous subset of cells that arise from unknown precursors in response to poorly understood cues. Here, we find that, in several models of liver autoimmunity, pharmacologically induced, autoantigen-specific T regulatory type-1 (TR1) cells and TR1-cell-induced B regulatory (Breg) cells use five immunoregulatory cytokines to coordinately recruit neutrophils into the liver and program their transcriptome to generate regulatory neutrophils. The liver-associated neutrophils from the treated mice, unlike their circulating counterparts or the liver neutrophils of sick mice lacking antigen-specific TR1 cells, are proliferative, can transfer disease protection to immunocompromised hosts engrafted with pathogenic effectors, and blunt antigen-presentation and local autoimmune responses via cathelin-related anti-microbial peptide (CRAMP), a cathelicidin, in a CRAMP-receptor-dependent manner. These results, thus, identify antigen-specific regulatory T cells as drivers of tissue-restricted regulatory neutrophil formation and CRAMP as an effector of regulatory neutrophil-mediated immunoregulation.
-
-
-
Immunology and Microbiology
GITR Agonism Triggers Antitumor Immune Responses through IL21-Expressing Follicular Helper T Cells.
In Cancer Immunol Res on 1 May 2020 by Koh, C. H., Kim, I. K., et al.
PubMed
Although treatment with the glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR) agonistic antibody (DTA-1) has shown antitumor activity in various tumor models, the underlying mechanism is not fully understood. Here, we demonstrate that interleukin (IL)-21-producing follicular helper T (Tfh) cells play a crucial role in DTA-1-induced tumor inhibition. The administration of DTA-1 increased IL21 expression by Tfh cells in an antigen-specific manner, and this activation led to enhanced antitumor cytotoxic T lymphocyte (CTL) activity. Mice treated with an antibody that neutralizes the IL21 receptor exhibited decreased antitumor activity when treated with DTA-1. Tumor growth inhibition by DTA-1 was abrogated in Bcl6fl/flCd4Cre mice, which are genetically deficient in Tfh cells. IL4 was required for optimal induction of IL21-expressing Tfh cells by GITR costimulation, and c-Maf mediated this pathway. Thus, our findings identify GITR costimulation as an inducer of IL21-expressing Tfh cells and provide a mechanism for the antitumor activity of GITR agonism.
-
-
-
Mus musculus (Mouse)
Orphan Nuclear Receptor NR2F6 Suppresses T Follicular Helper Cell Accumulation through Regulation of IL-21.
In Cell Rep on 10 September 2019 by Olson, W. J., Jakic, B., et al.
PubMed
CD4 T follicular helper (Tfh) cells are specialized in helping B cells during the germinal center (GC) reaction and ultimately promote long-term humoral immunity. Here we report that loss of the nuclear orphan receptor NR2F6 causes enhanced survival and accumulation of Tfh cells, GC B cells, and plasma cells (PCs) following T cell-dependent immunization. Nr2f6-deficient CD4 T cell dysfunction is the primary cause of cell accumulation. Cytokine expression in Nr2f6-deficient Tfh cells is dysregulated, and Il21 expression is enhanced. Mechanistically, NR2F6 binds directly to the interleukin 21 (IL-21) promoter and a conserved noncoding sequence (CNS) near the Il21 gene in resting CD4+ T cells. During Tfh cell differentiation, this direct NR2F6 DNA interaction is abolished. Enhanced Tfh cell accumulation in Nr2f6-deficient mice can be reverted by blocking IL-21R signaling. Thus, NR2F6 is a critical negative regulator of IL-21 cytokine production in Tfh cells and prevents excessive Tfh cell accumulation.
-
-
-
Immunology and Microbiology
Interleukin (IL)-21 promotes intestinal IgA response to microbiota.
In Mucosal Immunol on 1 September 2015 by Cao, A. T., Yao, S., et al.
PubMed
Commensal microbiota-specific T helper type 17 (Th17) cells are enriched in the intestines, which can convert into T follicular helper (Tfh) in Peyer's patches, and are crucial for production of intestinal immunoglobulin A (IgA) against microbiota; however, the role of Th17 and Tfh cytokines in regulating the mucosal IgA response to enteric microbiota is still not completely known. In this study, we found that intestinal IgA was impaired in mice deficient in interleukin (IL)-17 or IL-21 signaling. IL-21, but not IL-17, is able to augment B-cell differentiation to IgA(+) cells as mediated by transforming growth factor β1 (TGFβ1) and accelerate IgA class switch recombination (CSR). IL-21 and retinoic acid (RA) induce IgA(+) B-cell development and IgA production and drives autocrine TGFβ1 production to initiate IgA CSR. Repletion of T-cell-deficient TCRβxδ(-/-) mice with Th17 cells specific for commensal bacterial antigen increased the levels of IgA(+) B cells and IgA production in the intestine, which was blocked by neutralizing IL-21. Thus IL-21 functions to strongly augment IgA production under intestinal environment. Furthermore, IL-21 promotes intestinal B-cell homing through α4β7 expression, alone or with TGFβ and RA. Together, IL-21 from microbiota-specific Th17 and/or Tfh cells contributes to robust intestinal IgA levels by enhancing IgA(+) CSR, IgA production and B-cell trafficking into the intestine.
-