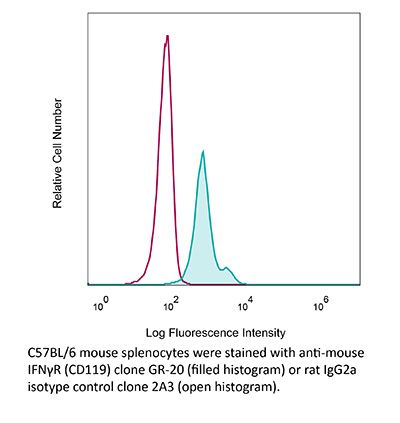
InVivoMAb anti-mouse IFNγR (CD119)
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | BALB/c mouse monomyelocytic cell line WEHI-3 |
| Reported Applications |
in vivo IFNγR neutralization in vitro IFNγR neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107576 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro IFNγR neutralization
van Montfoort, N., et al (2018). "NKG2A Blockade Potentiates CD8 T Cell Immunity Induced by Cancer Vaccines" Cell 175(7): 1744-1755 e1715.
PubMed
Tumor-infiltrating CD8 T cells were found to frequently express the inhibitory receptor NKG2A, particularly in immune-reactive environments and after therapeutic cancer vaccination. High-dimensional cluster analysis demonstrated that NKG2A marks a unique immune effector subset preferentially co-expressing the tissue-resident CD103 molecule, but not immune checkpoint inhibitors. To examine whether NKG2A represented an adaptive resistance mechanism to cancer vaccination, we blocked the receptor with an antibody and knocked out its ligand Qa-1(b), the conserved ortholog of HLA-E, in four mouse tumor models. The impact of therapeutic vaccines was greatly potentiated by disruption of the NKG2A/Qa-1(b) axis even in a PD-1 refractory mouse model. NKG2A blockade therapy operated through CD8 T cells, but not NK cells. These findings indicate that NKG2A-blocking antibodies might improve clinical responses to therapeutic cancer vaccines.
in vivo IFNγR neutralization
in vitro IFNγR neutralization
Wang, K. C., et al (2018). "Conserved and Differential Features of TNF Superfamily Ligand Expression on APC Subsets over the Course of a Chronic Viral Infection in Mice" Immunohorizons 2(11): 407-417.
PubMed
There is currently much interest in how different APC subsets shape the immune response. We recently described a division of labor between classical dendritic cells (cDC) and inflammatory monocyte-derived APC in provision of costimulatory ligands to T cells early during chronic lymphocytic choriomeningitis clone 13 (LCMV 13) infection in mice. At day 2 of LCMV 13 infection, cDC preferentially express CD80 and CD86, whereas TNF superfamily ligands GITRL, 4-1BBL, CD70, and OX40L are preferentially induced by type I IFN on inflammatory monocyte-derived APC, with minimal expression on cDC. In this study, we further investigate the expression of TNF and B7 family ligands on APC over the course of LCMV 13 infection. OX40L and 4-1BBL remain above baseline through the chronic stage of infection, with predominant expression on inflammatory APC compared with cDC in the spleen, partially blocked by anti-IFN-γR Ab pretreatment. Conversely, CD70, like GITRL, returns to baseline on the APC within a few days postinfection. In the lung, TNF family ligands were also preferentially expressed on inflammatory monocyte-derived APC. CD86 was generally higher on cDC than inflammatory APC in the spleen, but in the lung CD86 was highest on inflammatory APC. Moreover, in the spleen, CD80 levels on different APC subsets fluctuated over the course of the infection. We also show that LPS induction of TNF superfamily ligands is largely mediated through type I IFN. This study highlights the importance of IFNs and monocyte-derived APC in TNF superfamily ligand expression in both secondary lymphoid organs and tissues during chronic viral infection.
in vivo IFNγR neutralization
Vicetti Miguel, R. D., et al (2016). "Intravaginal Chlamydia trachomatis Challenge Infection Elicits TH1 and TH17 Immune Responses in Mice That Promote Pathogen Clearance and Genital Tract Damage" PLoS One 11(9): e0162445.
PubMed
While ascension of Chlamydia trachomatis into the upper genital tract of women can cause pelvic inflammatory disease and Fallopian tube damage, most infections elicit no symptoms or overt upper genital tract pathology. Consistent with this asymptomatic clinical presentation, genital C. trachomatis infection of women generates robust TH2 immunity. As an animal model that modeled this response would be invaluable for delineating bacterial pathogenesis and human host defenses, herein we explored if pathogen-specific TH2 immunity is similarly elicited by intravaginal (ivag) infection of mice with oculogenital C. trachomatis serovars. Analogous to clinical infection, ascension of primary C. trachomatis infection into the mouse upper genital tract produced no obvious tissue damage. Clearance of ivag challenge infection was mediated by interferon (IFN)-γ-producing CD4+ T cells, while IFN-γ signaling blockade concomitant with a single ivag challenge promoted tissue damage by enhancing Chlamydia-specific TH17 immunity. Likewise, IFN-γ and IL-17 signaling blockade or CD4+ T cell depletion eliminated the genital pathology produced in untreated controls by multiple ivag challenge infections. Conversely, we were unable to detect formation of pathogen-specific TH2 immunity in C. trachomatis-infected mice. Together, our work revealed C. trachomatis infection of mice generates TH1 and TH17 immune responses that promote pathogen clearance and immunopathological tissue damage. Absence of Chlamydia-specific TH2 immunity in these mice newly highlights the need to identify experimental models of C. trachomatis genital infection that more closely recapitulate the human host response.
in vivo IFNγR neutralization
Folias, A. E., et al (2014). "Aberrant innate immune activation following tissue injury impairs pancreatic regeneration" PLoS One 9(7): e102125.
PubMed
Normal tissue architecture is disrupted following injury, as resident tissue cells become damaged and immune cells are recruited to the site of injury. While injury and inflammation are critical to tissue remodeling, the inability to resolve this response can lead to the destructive complications of chronic inflammation. In the pancreas, acinar cells of the exocrine compartment respond to injury by transiently adopting characteristics of progenitor cells present during embryonic development. This process of de-differentiation creates a window where a mature and stable cell gains flexibility and is potentially permissive to changes in cellular fate. How de-differentiation can turn an acinar cell into another cell type (such as a pancreatic beta-cell), or a cell with cancerous potential (as in cases of deregulated Kras activity) is of interest to both the regenerative medicine and cancer communities. While it is known that inflammation and acinar de-differentiation increase following pancreatic injury, it remains unclear which immune cells are involved in this process. We used a combination of genetically modified mice, immunological blockade and cellular characterization to identify the immune cells that impact pancreatic regeneration in an in vivo model of pancreatitis. We identified the innate inflammatory response of macrophages and neutrophils as regulators of pancreatic regeneration. Under normal conditions, mild innate inflammation prompts a transient de-differentiation of acinar cells that readily dissipates to allow normal regeneration. However, non-resolving inflammation developed when elevated pancreatic levels of neutrophils producing interferon-gamma increased iNOS levels and the pro-inflammatory response of macrophages. Pancreatic injury improved following in vivo macrophage depletion, iNOS inhibition as well as suppression of iNOS levels in macrophages via interferon-gamma blockade, supporting the impairment in regeneration and the development of chronic inflammation arises from aberrant activation of the innate inflammatory response. Collectively these studies identify targetable inflammatory factors that can be used to influence the development of non-resolving inflammation and pancreatic regeneration following injury.
Product Citations
-
-
Immunology and Microbiology
An interleukin-27-centered cytokine circuit regulates macrophage and T cell interactions in autoimmune diabetes.
In iScience on 17 October 2025 by Ciecko, A. E., Nabi, R., et al.
PubMed
In the non-obese diabetic (NOD) mouse model of autoimmune diabetes, interleukin (IL)-27 stimulates interferon γ (IFNγ) production by CD4 and CD8 T cells and is essential for disease development. Here, we tested the role of IL-27 in cellular communication. Single-cell RNA sequencing and T cell adoptive transfer showed that IL-27 intrinsically controlled the differentiation of islet-infiltrating CD4 T cells by driving them toward an IL-21+ Th1 phenotype. Consequently, IL-27 signaling in CD4 T cells was important for BATF and granzyme B expression in islet CD8 T effectors. BATF overexpression increased the diabetogenic potential of β cell autoreactive CD8 T cells lacking help from CD4 T cell-derived IL-21. Macrophages were the main source of IL-27 in the islets, whose expression correlated with T cell infiltration. IFNγ and CD40 signaling conferred by activated T cells induced macrophage IL-27 production. Collectively, our findings reveal a role for IL-27 in orchestrating interconnected positive feedback loops involving CD4 T cells, CD8 T cells, and macrophages in autoimmune diabetes.
-
-
-
Immunology and Microbiology
IFN-γ signaling links ventriculomegaly to choroid plexus and ependyma dysfunction following maternal immune activation.
In J Neuroinflammation on 15 March 2025 by Sun, Y. Q., Huang, X. X., et al.
PubMed
Maternal immune activation (MIA) is a principal environmental risk factor contributing to autism spectrum disorder (ASD) and can be causally linked to ASD symptoms. In our study, we found that MIA triggered by poly (I: C) injection caused ventriculomegaly in offspring due to the dysfunction of the choroid plexus (Chp) and ependyma. We subsequently identified a sustained enhancement of interferon-γ (IFN-γ) signaling in the brain and serum of MIA offspring. Further study revealed that increased IFN-γ signaling could disrupt the barrier function of Chp epithelial cells by activating macrophages, and suppress the differentiation of primary ependymal cells via the signal transducer and activator of transcription 1/3 signaling. The effects of MIA on the offspring were mitigated by administration of IFNGR-blocking antibody in pregnant dams, while systemic maternal administration of IFN-γ was sufficient to mimic the effect of MIA. Overall, our findings revealed that ventriculomegaly caused by IFN-γ signaling could be a critical factor in compromising fetal brain development in MIA-induced ASD and provide a mechanistic framework for the association between maternal inflammation and abnormal development of ventricles in the offspring.
-
-
-
Mus musculus (Mouse)
-
Cardiovascular biology
-
Immunology and Microbiology
Lipopolysaccharide Induces Trained Innate Immune Tolerance in the Heart Through Interferon Signaling in a Model of Stress-Induced Cardiomyopathy
In bioRxiv on 26 September 2024 by Lim, K. R. Q., Amrute, J. M., et al.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Interferon-stimulated neutrophils as a predictor of immunotherapy response.
In Cancer Cell on 12 February 2024 by Benguigui, M., Cooper, T. J., et al.
PubMed
Despite the remarkable success of anti-cancer immunotherapy, its effectiveness remains confined to a subset of patients-emphasizing the importance of predictive biomarkers in clinical decision-making and further mechanistic understanding of treatment response. Current biomarkers, however, lack the power required to accurately stratify patients. Here, we identify interferon-stimulated, Ly6Ehi neutrophils as a blood-borne biomarker of anti-PD1 response in mice at baseline. Ly6Ehi neutrophils are induced by tumor-intrinsic activation of the STING (stimulator of interferon genes) signaling pathway and possess the ability to directly sensitize otherwise non-responsive tumors to anti-PD1 therapy, in part through IL12b-dependent activation of cytotoxic T cells. By translating our pre-clinical findings to a cohort of patients with non-small cell lung cancer and melanoma (n = 109), and to public data (n = 1440), we demonstrate the ability of Ly6Ehi neutrophils to predict immunotherapy response in humans with high accuracy (average AUC ≈ 0.9). Overall, our study identifies a functionally active biomarker for use in both mice and humans.
-
-
-
Immunology and Microbiology
Natural Killer Cell-Derived Interferon-γ Regulates Macrophage-Mediated Immunopathology During Viral Infection.
In J Infect Dis on 3 October 2023 by Feng, E., Monteiro, J. K., et al.
PubMed
Regulation of immune responses during viral infection is critical to preventing the development immunopathology that impairs host survival. Natural killer (NK) cells are well known for their antiviral functions that promote viral clearance; however, their roles in limiting immune-mediated pathology are still unclear. Using a mouse model for genital herpes simplex virus type 2 infection, we find that NK cell-derived interferon-γ directly counteracts interleukin-6-mediated matrix metalloproteases (MMPs) activity in macrophages to limit MMP-mediated tissue damage. Our findings uncover a key immunoregulatory function of NK cells during host-pathogen interactions that highlight the potential of NK cell therapy for treatment of severe viral infections.
-
-
-
Immunology and Microbiology
Active eosinophils regulate host defence and immune responses in colitis.
In Nature on 1 March 2023 by Gurtner, A., Borrelli, C., et al.
PubMed
In the past decade, single-cell transcriptomics has helped to uncover new cell types and states and led to the construction of a cellular compendium of health and disease. Despite this progress, some difficult-to-sequence cells remain absent from tissue atlases. Eosinophils-elusive granulocytes that are implicated in a plethora of human pathologies1-5-are among these uncharted cell types. The heterogeneity of eosinophils and the gene programs that underpin their pleiotropic functions remain poorly understood. Here we provide a comprehensive single-cell transcriptomic profiling of mouse eosinophils. We identify an active and a basal population of intestinal eosinophils, which differ in their transcriptome, surface proteome and spatial localization. By means of a genome-wide CRISPR inhibition screen and functional assays, we reveal a mechanism by which interleukin-33 (IL-33) and interferon-γ (IFNγ) induce the accumulation of active eosinophils in the inflamed colon. Active eosinophils are endowed with bactericidal and T cell regulatory activity, and express the co-stimulatory molecules CD80 and PD-L1. Notably, active eosinophils are enriched in the lamina propria of a small cohort of patients with inflammatory bowel disease, and are closely associated with CD4+ T cells. Our findings provide insights into the biology of eosinophils and highlight the crucial contribution of this cell type to intestinal homeostasis, immune regulation and host defence. Furthermore, we lay a framework for the characterization of eosinophils in human gastrointestinal diseases.
-
-
-
In vitro experiments
-
In vitro experiments
-
In vitro experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Conserved and Differential Features of TNF Superfamily Ligand Expression on APC Subsets over the Course of a Chronic Viral Infection in Mice.
In Immunohorizons on 20 December 2018 by Wang, K. C., Chu, K. L., et al.
PubMed
There is currently much interest in how different APC subsets shape the immune response. We recently described a division of labor between classical dendritic cells (cDC) and inflammatory monocyte-derived APC in provision of costimulatory ligands to T cells early during chronic lymphocytic choriomeningitis clone 13 (LCMV 13) infection in mice. At day 2 of LCMV 13 infection, cDC preferentially express CD80 and CD86, whereas TNF superfamily ligands GITRL, 4-1BBL, CD70, and OX40L are preferentially induced by type I IFN on inflammatory monocyte-derived APC, with minimal expression on cDC. In this study, we further investigate the expression of TNF and B7 family ligands on APC over the course of LCMV 13 infection. OX40L and 4-1BBL remain above baseline through the chronic stage of infection, with predominant expression on inflammatory APC compared with cDC in the spleen, partially blocked by anti-IFN-γR Ab pretreatment. Conversely, CD70, like GITRL, returns to baseline on the APC within a few days postinfection. In the lung, TNF family ligands were also preferentially expressed on inflammatory monocyte-derived APC. CD86 was generally higher on cDC than inflammatory APC in the spleen, but in the lung CD86 was highest on inflammatory APC. Moreover, in the spleen, CD80 levels on different APC subsets fluctuated over the course of the infection. We also show that LPS induction of TNF superfamily ligands is largely mediated through type I IFN. This study highlights the importance of IFNs and monocyte-derived APC in TNF superfamily ligand expression in both secondary lymphoid organs and tissues during chronic viral infection.
-
-
-
Blocking experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
NKG2A Blockade Potentiates CD8 T Cell Immunity Induced by Cancer Vaccines.
In Cell on 13 December 2018 by van Montfoort, N., Borst, L., et al.
PubMed
Tumor-infiltrating CD8 T cells were found to frequently express the inhibitory receptor NKG2A, particularly in immune-reactive environments and after therapeutic cancer vaccination. High-dimensional cluster analysis demonstrated that NKG2A marks a unique immune effector subset preferentially co-expressing the tissue-resident CD103 molecule, but not immune checkpoint inhibitors. To examine whether NKG2A represented an adaptive resistance mechanism to cancer vaccination, we blocked the receptor with an antibody and knocked out its ligand Qa-1b, the conserved ortholog of HLA-E, in four mouse tumor models. The impact of therapeutic vaccines was greatly potentiated by disruption of the NKG2A/Qa-1b axis even in a PD-1 refractory mouse model. NKG2A blockade therapy operated through CD8 T cells, but not NK cells. These findings indicate that NKG2A-blocking antibodies might improve clinical responses to therapeutic cancer vaccines.
-
-
-
Immunodepletion
-
Immunodepletion
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Intravaginal Chlamydia trachomatis Challenge Infection Elicits TH1 and TH17 Immune Responses in Mice That Promote Pathogen Clearance and Genital Tract Damage.
In PLoS One on 9 September 2016 by Vicetti Miguel, R. D., Quispe Calla, N. E., et al.
PubMed
While ascension of Chlamydia trachomatis into the upper genital tract of women can cause pelvic inflammatory disease and Fallopian tube damage, most infections elicit no symptoms or overt upper genital tract pathology. Consistent with this asymptomatic clinical presentation, genital C. trachomatis infection of women generates robust TH2 immunity. As an animal model that modeled this response would be invaluable for delineating bacterial pathogenesis and human host defenses, herein we explored if pathogen-specific TH2 immunity is similarly elicited by intravaginal (ivag) infection of mice with oculogenital C. trachomatis serovars. Analogous to clinical infection, ascension of primary C. trachomatis infection into the mouse upper genital tract produced no obvious tissue damage. Clearance of ivag challenge infection was mediated by interferon (IFN)-γ-producing CD4+ T cells, while IFN-γ signaling blockade concomitant with a single ivag challenge promoted tissue damage by enhancing Chlamydia-specific TH17 immunity. Likewise, IFN-γ and IL-17 signaling blockade or CD4+ T cell depletion eliminated the genital pathology produced in untreated controls by multiple ivag challenge infections. Conversely, we were unable to detect formation of pathogen-specific TH2 immunity in C. trachomatis-infected mice. Together, our work revealed C. trachomatis infection of mice generates TH1 and TH17 immune responses that promote pathogen clearance and immunopathological tissue damage. Absence of Chlamydia-specific TH2 immunity in these mice newly highlights the need to identify experimental models of C. trachomatis genital infection that more closely recapitulate the human host response.
-
-
-
Immunology and Microbiology
Aberrant innate immune activation following tissue injury impairs pancreatic regeneration.
In PLoS One on 11 July 2014 by Folias, A. E., Penaranda, C., et al.
PubMed
Normal tissue architecture is disrupted following injury, as resident tissue cells become damaged and immune cells are recruited to the site of injury. While injury and inflammation are critical to tissue remodeling, the inability to resolve this response can lead to the destructive complications of chronic inflammation. In the pancreas, acinar cells of the exocrine compartment respond to injury by transiently adopting characteristics of progenitor cells present during embryonic development. This process of de-differentiation creates a window where a mature and stable cell gains flexibility and is potentially permissive to changes in cellular fate. How de-differentiation can turn an acinar cell into another cell type (such as a pancreatic β-cell), or a cell with cancerous potential (as in cases of deregulated Kras activity) is of interest to both the regenerative medicine and cancer communities. While it is known that inflammation and acinar de-differentiation increase following pancreatic injury, it remains unclear which immune cells are involved in this process. We used a combination of genetically modified mice, immunological blockade and cellular characterization to identify the immune cells that impact pancreatic regeneration in an in vivo model of pancreatitis. We identified the innate inflammatory response of macrophages and neutrophils as regulators of pancreatic regeneration. Under normal conditions, mild innate inflammation prompts a transient de-differentiation of acinar cells that readily dissipates to allow normal regeneration. However, non-resolving inflammation developed when elevated pancreatic levels of neutrophils producing interferon-γ increased iNOS levels and the pro-inflammatory response of macrophages. Pancreatic injury improved following in vivo macrophage depletion, iNOS inhibition as well as suppression of iNOS levels in macrophages via interferon-γ blockade, supporting the impairment in regeneration and the development of chronic inflammation arises from aberrant activation of the innate inflammatory response. Collectively these studies identify targetable inflammatory factors that can be used to influence the development of non-resolving inflammation and pancreatic regeneration following injury.
-