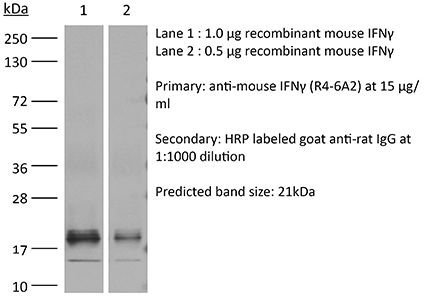
InVivoMAb anti-mouse IFNγ
Product Description
Specifications
| Isotype | Rat IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase |
| Recommended Dilution Buffer | InVivoPure pH 8.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Partially-purified native mouse IFNγ |
| Reported Applications |
in vivo IFNγ neutralization in vitro IFNγ neutralization |
| Formulation |
PBS, pH 8.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107692 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo IFNγ neutralization
Clemente-Casares, X., et al (2016). "Expanding antigen-specific regulatory networks to treat autoimmunity" Nature 530(7591): 434-440.
PubMed
Regulatory T cells hold promise as targets for therapeutic intervention in autoimmunity, but approaches capable of expanding antigen-specific regulatory T cells in vivo are currently not available. Here we show that systemic delivery of nanoparticles coated with autoimmune-disease-relevant peptides bound to major histocompatibility complex class II (pMHCII) molecules triggers the generation and expansion of antigen-specific regulatory CD4(+) T cell type 1 (TR1)-like cells in different mouse models, including mice humanized with lymphocytes from patients, leading to resolution of established autoimmune phenomena. Ten pMHCII-based nanomedicines show similar biological effects, regardless of genetic background, prevalence of the cognate T-cell population or MHC restriction. These nanomedicines promote the differentiation of disease-primed autoreactive T cells into TR1-like cells, which in turn suppress autoantigen-loaded antigen-presenting cells and drive the differentiation of cognate B cells into disease-suppressing regulatory B cells, without compromising systemic immunity. pMHCII-based nanomedicines thus represent a new class of drugs, potentially useful for treating a broad spectrum of autoimmune conditions in a disease-specific manner.
in vivo IFNγ neutralization
Conde, P., et al (2015). "DC-SIGN(+) Macrophages Control the Induction of Transplantation Tolerance" Immunity 42(6): 1143-1158.
PubMed
Tissue effector cells of the monocyte lineage can differentiate into different cell types with specific cell function depending on their environment. The phenotype, developmental requirements, and functional mechanisms of immune protective macrophages that mediate the induction of transplantation tolerance remain elusive. Here, we demonstrate that costimulatory blockade favored accumulation of DC-SIGN-expressing macrophages that inhibited CD8(+) T cell immunity and promoted CD4(+)Foxp3(+) Treg cell expansion in numbers. Mechanistically, that simultaneous DC-SIGN engagement by fucosylated ligands and TLR4 signaling was required for production of immunoregulatory IL-10 associated with prolonged allograft survival. Deletion of DC-SIGN-expressing macrophages in vivo, interfering with their CSF1-dependent development, or preventing the DC-SIGN signaling pathway abrogated tolerance. Together, the results provide new insights into the tolerogenic effects of costimulatory blockade and identify DC-SIGN(+) suppressive macrophages as crucial mediators of immunological tolerance with the concomitant therapeutic implications in the clinic.
in vitro IFNγ neutralization
Wensveen, F. M., et al (2015). "NK cells link obesity-induced adipose stress to inflammation and insulin resistance" Nat Immunol 16(4): 376-385.
PubMed
An important cause of obesity-induced insulin resistance is chronic systemic inflammation originating in visceral adipose tissue (VAT). VAT inflammation is associated with the accumulation of proinflammatory macrophages in adipose tissue, but the immunological signals that trigger their accumulation remain unknown. We found that a phenotypically distinct population of tissue-resident natural killer (NK) cells represented a crucial link between obesity-induced adipose stress and VAT inflammation. Obesity drove the upregulation of ligands of the NK cell-activating receptor NCR1 on adipocytes; this stimulated NK cell proliferation and interferon-gamma (IFN-gamma) production, which in turn triggered the differentiation of proinflammatory macrophages and promoted insulin resistance. Deficiency of NK cells, NCR1 or IFN-gamma prevented the accumulation of proinflammatory macrophages in VAT and greatly ameliorated insulin sensitivity. Thus NK cells are key regulators of macrophage polarization and insulin resistance in response to obesity-induced adipocyte stress.
in vivo IFNγ neutralization
Deligne, C., et al (2015). "Anti-CD20 therapy induces a memory Th1 response through the IFN-gamma/IL-12 axis and prevents protumor regulatory T-cell expansion in mice" Leukemia 29(4): 947-957.
PubMed
The long-lasting clinical response by lymphoma patients to anti-CD20 therapy has been attributed to the induction of an anti-tumor adaptive immunity. We previously demonstrated that a CD4-dependent mechanism is responsible for the long-term protection of CD20(+) tumor-bearing mice by anti-CD20 treatment. Here, we compare tumor immunity in tumor-bearing animals that did or did not receive anti-CD20 treatment. Splenic CD4(+)FoxP3(+) regulatory T cells (Tregs) expanded substantially in untreated mice that exhibited then a reduced survival, whereas Tregs depletion led to long-term survival of the animals, suggesting the establishment of a Treg-dependent immunosuppressive environment after tumor injection. Strikingly, anti-CD20 therapy reversed the initial expansion of Tregs, and was accompanied by a marked increase in the number of Th1 cells, with no detectable change in Th2 and Th17 cell numbers. Interleukin-12 serum level was also increased by the anti-CD20 treatment, and activated myeloid dendritic cells producing interleukin-12 could be detected in lymph nodes of treated animals, while interferon-gamma blockade strongly reduced survival. Also, CD4(+) effector memory T cells were evidenced in surviving animals, and the transfer of CD4(+) T cells induced long-term protection. Thus, anti-CD20 therapy promotes strong anti-tumor adaptive immunity, opposes Treg expansion and inhibits tumor cells from maintaining an immunosuppressive environment.
in vivo IFNγ neutralization
Maltby, S., et al (2014). "Production and differentiation of myeloid cells driven by proinflammatory cytokines in response to acute pneumovirus infection in mice" J Immunol 193(8): 4072-4082.
PubMed
Respiratory virus infections are often pathogenic, driving severe inflammatory responses. Most research has focused on localized effects of virus infection and inflammation. However, infection can induce broad-reaching, systemic changes that are only beginning to be characterized. In this study, we assessed the impact of acute pneumovirus infection in C57BL/6 mice on bone marrow hematopoiesis. We hypothesized that inflammatory cytokine production in the lung upregulates myeloid cell production in response to infection. We demonstrate a dramatic increase in the percentages of circulating myeloid cells, which is associated with pronounced elevations in inflammatory cytokines in serum (IFN-gamma, IL-6, CCL2), bone (TNF-alpha), and lung tissue (TNF-alpha, IFN-gamma, IL-6, CCL2, CCL3, G-CSF, osteopontin). Increased hematopoietic stem/progenitor cell percentages (Lineage(-)Sca-I(+)c-kit(+)) were also detected in the bone marrow. This increase was accompanied by an increase in the proportions of committed myeloid progenitors, as determined by colony-forming unit assays. However, no functional changes in hematopoietic stem cells occurred, as assessed by competitive bone marrow reconstitution. Systemic administration of neutralizing Abs to either TNF-alpha or IFN-gamma blocked expansion of myeloid progenitors in the bone marrow and also limited virus clearance from the lung. These findings suggest that acute inflammatory cytokines drive production and differentiation of myeloid cells in the bone marrow by inducing differentiation of committed myeloid progenitors. Our findings provide insight into the mechanisms via which innate immune responses regulate myeloid cell progenitor numbers in response to acute respiratory virus infection.
in vivo IFNγ neutralization
Walsh, K. B., et al (2014). "Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy" J Virol 88(11): 6281-6293.
PubMed
The cytokine storm is an intensified, dysregulated, tissue-injurious inflammatory response driven by cytokine and immune cell components. The cytokine storm during influenza virus infection, whereby the amplified innate immune response is primarily responsible for pulmonary damage, has been well characterized. Now we describe a novel event where virus-specific T cells induce a cytokine storm. The paramyxovirus pneumonia virus of mice (PVM) is a model of human respiratory syncytial virus (hRSV). Unexpectedly, when C57BL/6 mice were infected with PVM, the innate inflammatory response was undetectable until day 5 postinfection, at which time CD8(+) T cells infiltrated into the lung, initiating a cytokine storm by their production of gamma interferon (IFN-gamma) and tumor necrosis factor alpha (TNF-alpha). Administration of an immunomodulatory sphingosine-1-phosphate (S1P) receptor 1 (S1P1R) agonist significantly inhibited PVM-elicited cytokine storm by blunting the PVM-specific CD8(+) T cell response, resulting in diminished pulmonary disease and enhanced survival. IMPORTANCE: A dysregulated overly exuberant immune response, termed a “cytokine storm,” accompanies virus-induced acute respiratory diseases (VARV), is primarily responsible for the accompanying high morbidity and mortality, and can be controlled therapeutically in influenza virus infection of mice and ferrets by administration of sphingosine-1-phosphate 1 receptor (S1P1R) agonists. Here, two novel findings are recorded. First, in contrast to influenza infection, where the cytokine storm is initiated early by the innate immune system, for pneumonia virus of mice (PVM), a model of RSV, the cytokine storm is initiated late in infection by the adaptive immune response: specifically, by virus-specific CD8 T cells via their release of IFN-gamma and TNF-alpha. Blockading these cytokines with neutralizing antibodies blunts the cytokine storm and protects the host. Second, PVM infection is controlled by administration of an S1P1R agonist.
in vivo IFNγ neutralization
Hock, K., et al (2014). "Donor CD4 T cells trigger costimulation blockade-resistant donor bone marrow rejection through bystander activation requiring IL-6" Am J Transplant 14(9): 2011-2022.
PubMed
Bone marrow (BM) transplantation under costimulation blockade induces chimerism and tolerance. Cotransplantation of donor T cells (contained in substantial numbers in mobilized peripheral blood stem cells and donor lymphocyte infusions) together with donor BM paradoxically triggers rejection of donor BM through undefined mechanisms. Here, nonmyeloablatively irradiated C57BL/6 recipients simultaneously received donor BM (BALB/c) and donor T cells under costimulation blockade (anti-CD154 and CTLA4Ig). Donor CD4, but not CD8 cells, triggered natural killer-independent donor BM rejection which was associated with increased production of IL-6, interferon gamma (IFN-gamma) and IL-17A. BM rejection was prevented through neutralization of IL-6, but not of IFN-gamma or IL-17A. IL-6 counteracted the antiproliferative effect of anti-CD154 in vitro. Rapamycin and anti-lymphocyte function-associated antigen 1 negated this effect of IL-6 in vitro and prevented BM rejection in vivo. Simultaneous cotransplantation of (BALB/cxB6)F1, recipient or irradiated donor CD4 cells, or late transfer of donor CD4 cells did not lead to BM rejection, whereas cotransplantation of third party CD4 cells did. Transferred donor CD4 cells became activated, rapidly underwent apoptosis and triggered activation and proliferation of recipient T cells. Collectively, these results provide evidence that donor T cells recognizing the recipient as allogeneic lead to the release of IL-6, which abolishes the effect of anti-CD154, triggering donor BM rejection through bystander activation.
in vivo IFNγ neutralization
Choi, I. K., et al (2013). "Oncolytic adenovirus expressing IL-23 and p35 elicits IFN-gamma- and TNF-alpha-co-producing T cell-mediated antitumor immunity" PLoS One 8(7): e67512.
PubMed
Cytokine immunogene therapy is a promising strategy for cancer treatment. Interleukin (IL)-12 boosts potent antitumor immunity by inducing T helper 1 cell differentiation and stimulating cytotoxic T lymphocyte and natural killer cell cytotoxicity. IL-23 has been proposed to have similar but not overlapping functions with IL-12 in inducing Th1 cell differentiation and antitumor immunity. However, the therapeutic effects of intratumoral co-expression of IL-12 and IL-23 in a cancer model have yet to be investigated. Therefore, we investigated for the first time an effective cancer immunogene therapy of syngeneic tumors via intratumoral inoculation of oncolytic adenovirus co-expressing IL-23 and p35, RdB/IL23/p35. Intratumoral administration of RdB/IL23/p35 elicited strong antitumor effects and increased survival in a murine B16-F10 syngeneic tumor model. The levels of IL-12, IL-23, interferon-gamma (IFN-gamma), and tumor necrosis factor-alpha (TNF-alpha) were elevated in RdB/IL23/p35-treated tumors. Moreover, the proportion of regulatory T cells was markedly decreased in mice treated with RdB/IL23/p35. Consistent with these data, mice injected with RdB/IL23/p35 showed massive infiltration of CD4(+) and CD8(+) T cells into the tumor as well as enhanced induction of tumor-specific immunity. Importantly, therapeutic mechanism of antitumor immunity mediated by RdB/IL23/p35 is associated with the generation and recruitment of IFN-gamma- and TNF-alpha-co-producing T cells in tumor microenvironment. These results provide a new insight into therapeutic mechanisms of IL-12 plus IL-23 and provide a potential clinical cancer immunotherapeutic agent for improved antitumor immunity.
in vitro IFNγ neutralization
Wu, H. Y., et al (2011). "In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling" PLoS One 6(8): e23618.
PubMed
BACKGROUND: Type 1 regulatory T (Tr1) cells, characterized by the secretion of high levels of the anti-inflammatory cytokine interleukin-10 (IL-10), play an important role in the regulation of autoimmune diseases and transplantation. However, effective strategies that specifically induce Tr1 cells in vivo are limited. Furthermore, the pathways controlling the induction of these cells in vivo are not well understood. METHODOLOGY/PRINCIPAL FINDINGS: Here we report that nasal administration of anti-CD3 antibody induces suppressive Tr1 cells in mice. The in vivo induction of Tr1 cells by nasal anti-CD3 is dependent on IL-27 produced by upper airway resident dendritic cells (DCs), and is controlled by the transcription factors aryl hydrocarbon receptor (AHR) and c-Maf. Subsequently, IL-21 acts in an autocrine fashion to expand and maintain the Tr1 cells induced in vivo by nasally administered anti-CD3. CONCLUSIONS/SIGNIFICANCE: Our findings identify a unique approach to generate Tr1 cells in vivo and provide insights into the mechanisms by which these cells are induced.
in vivo IFNγ neutralization
Coley, S. M., et al (2009). "IFN-gamma dictates allograft fate via opposing effects on the graft and on recipient CD8 T cell responses" J Immunol 182(1): 225-233.
PubMed
CD8 T cells are necessary for costimulation blockade-resistant rejection. However, the mechanism by which CD8 T cells mediate rejection in the absence of major costimulatory signals is poorly understood. IFN-gamma promotes CD8 T cell-mediated immune responses, but IFN-gamma-deficient mice show early graft loss despite costimulation blockade. In contrast, we found that IFN-gamma receptor knockout mice show dramatically prolonged graft survival under costimulation blockade. To investigate this paradox, we addressed the effects of IFN-gamma on T cell alloresponses in vivo independent of the effects of IFN-gamma on graft survival. We identified a donor-specific CD8 T cell breakthrough response temporally correlated with costimulation blockade-resistant rejection. Neither IFN-gamma receptor knockout recipients nor IFN-gamma-deficient recipients showed a CD8 breakthrough response. Graft death on IFN-gamma-deficient recipients despite costimulation blockade could be explained by the lack of IFN-gamma available to act on the graft. Indeed, the presence of IFN-gamma was necessary for graft survival on IFN-gamma receptor knockout recipients, as either IFN-gamma neutralization or the lack of the IFN-gamma receptor on the graft precipitated early graft loss. Thus, IFN-gamma is required both for the recipient to mount a donor-specific CD8 T cell response under costimulation blockade as well as for the graft to survive after allotransplantation.
Product Citations
-
-
Cancer Research
Sorafenib-treated Th9 cells exhibit superior anti-tumor effect in lung metastasis.
In Sci Rep on 7 August 2025 by Yuan, F., Wang, H., et al.
PubMed
The lung is the second most common organ of tumor metastasis, with limited treatment options and disappointing treatment outcomes. T helper 9 (Th9) cells have been reported to be effective in the elimination of solid tumors and exhibit superior antitumor properties to T helper 1 (Th1) and T helper 17 (Th17) cells, which makes a potential candidate for Adoptive cell therapy (ACT) against lung metastasis. However, how Th9 cells can be massively expanded in vitro is not clear. Here, we found Sorafenib (Sora) can increase the efficiency of Th9 in vitro through ERK signaling. In addition, Sora-treated Th9 cells exhibit superior anti-tumor effects by reshaping tumor immune microenvironment in lung metastasis mice model. Thus, our findings provide a potential method to expand Th9 cells in vitro for lung metastasis treatment.
-
-
The emerging fungal pathogenCandida aurisinduces IFNγ to colonize mammalian hair follicles
In bioRxiv on 18 January 2025 by Merrill, E. D., Prudent, V., et al.
-
-
Immunology and Microbiology
Pathobiont-induced suppressive immune imprints thwart T cell vaccine responses.
In Nat Commun on 16 December 2024 by Hajam, I. A., Tsai, C. M., et al.
PubMed
Pathobionts have evolved many strategies to coexist with the host, but how immune evasion mechanisms contribute to the difficulty of developing vaccines against pathobionts is unclear. Meanwhile, Staphylococcus aureus (SA) has resisted human vaccine development to date. Here we show that prior SA exposure induces non-protective CD4+ T cell imprints, leading to the blunting of protective IsdB vaccine responses. Mechanistically, these SA-experienced CD4+ T cells express IL-10, which is further amplified by vaccination and impedes vaccine protection by binding with IL-10Rα on CD4+ T cell and inhibit IL-17A production. IL-10 also mediates cross-suppression of IsdB and sdrE multi-antigen vaccine. By contrast, the inefficiency of SA IsdB, IsdA and MntC vaccines can be overcome by co-treatment with adjuvants that promote IL-17A and IFN-γ responses. We thus propose that IL-10 secreting, SA-experienced CD4+ T cell imprints represent a staphylococcal immune escaping mechanism that needs to be taken into consideration for future vaccine development.
-
-
Natural lung-tropic TH9 cells: a sharp weapon for established lung metastases.
In J Immunother Cancer on 4 December 2024 by Chen, T., Qiao, C., et al.
PubMed
Lung metastasis remains the primary cause of tumor-related mortality, with limited treatment options and unsatisfactory efficacy. In preclinical studies, T helper 9 (TH9) cells have shown promise in treating solid tumors. However, it is unclear whether TH9 cells can tackle more challenging situations, such as established lung metastases. Moreover, comprehensive exploration into the nuanced biological attributes of TH9 cells is imperative to further unravel their therapeutic potential.
-
-
Cancer Research
-
Immunology and Microbiology
A CXCR4 partial agonist improves immunotherapy by targeting polymorphonuclear myeloid-derived suppressor cells and cancer-driven granulopoiesis
In bioRxiv on 11 October 2024 by Qian, J., Ma, C., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting TNFRSF25 by agonistic antibodies and multimeric TL1A proteins co-stimulated CD8+ T cells and inhibited tumor growth.
In J Immunother Cancer on 13 August 2024 by Lyu, X., Zhao, L., et al.
PubMed
Tumor necrosis factor receptor superfamily 25 (TNFRSF25) is a T-cell co-stimulatory receptor. Expression of its ligand, TNF-like cytokine 1A (TL1A), on mouse tumor cells has been shown to promote tumor regression. This study aimed to develop TNFRSF25 agonists (both antibodies (Abs) and TL1A proteins) and to investigate their potential antitumor effects.
-
-
-
Neuroscience
-
Immunohistochemistry
-
Immunohistochemistry
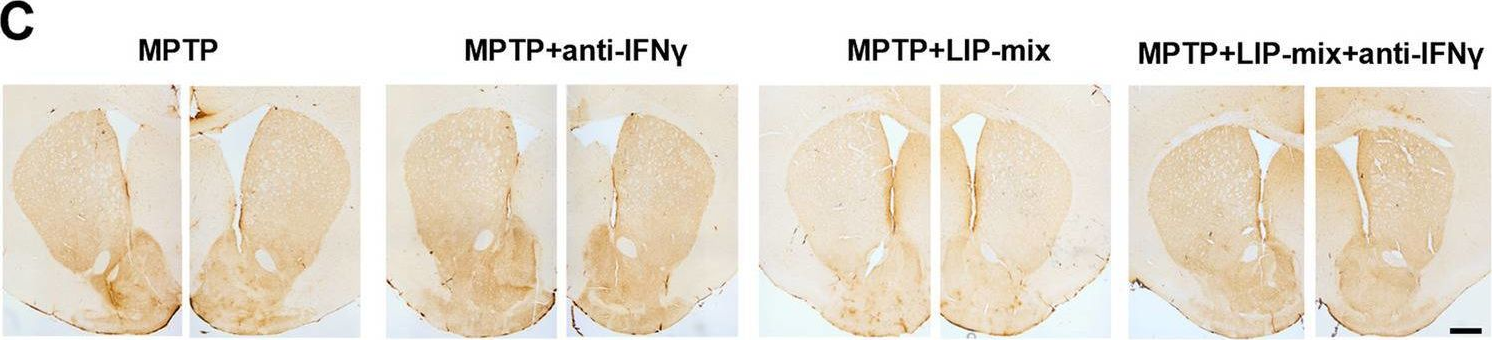
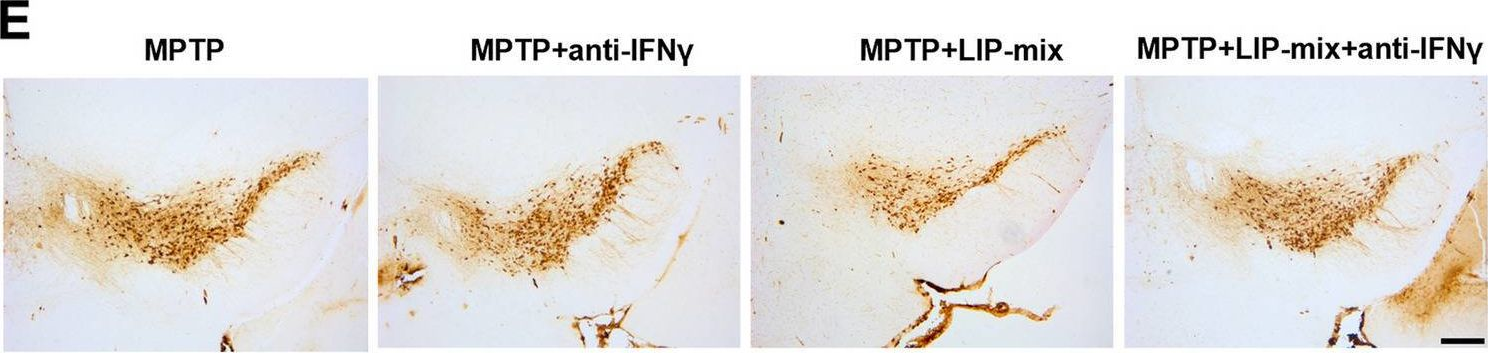
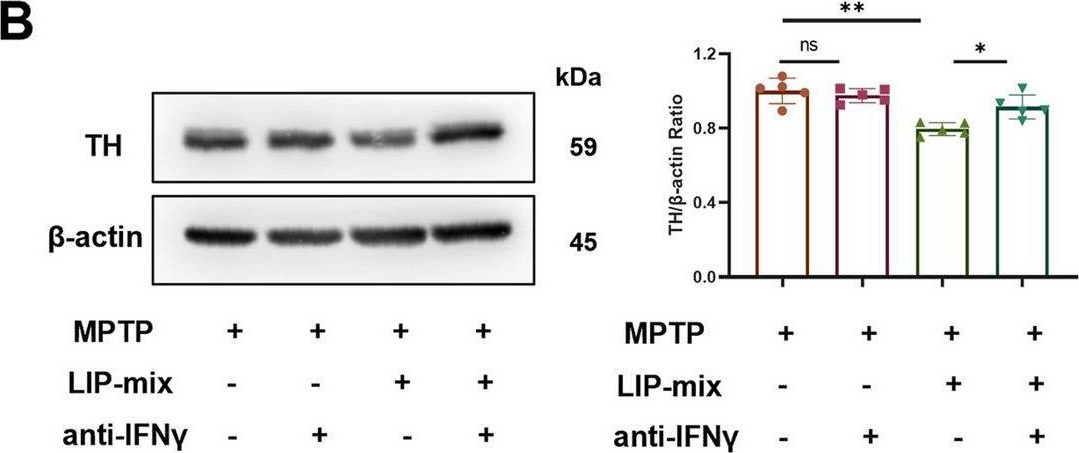
Oral pathogens exacerbate Parkinson's disease by promoting Th1 cell infiltration in mice.
In Microbiome on 17 November 2023 by Bai, X. B., Xu, S., et al.
PubMed
Parkinson's disease (PD) is a common chronic neurological disorder with a high risk of disability and no cure. Periodontitis is an infectious bacterial disease occurring in periodontal supporting tissues. Studies have shown that periodontitis is closely related to PD. However, direct evidence of the effect of periodontitis on PD is lacking. Here, we demonstrated that ligature-induced periodontitis with application of subgingival plaque (LIP-SP) exacerbated motor dysfunction, microglial activation, and dopaminergic neuron loss in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice.
-
-
-
Immunology and Microbiology
RORγt-Raftlin1 complex regulates the pathogenicity of Th17 cells and colonic inflammation.
In Nat Commun on 17 August 2023 by Singh, A. K., Kumar, R., et al.
PubMed
Th17 cells that produce Interleukin IL-17 are pathogenic in many human diseases, including inflammatory bowel disease, but are, paradoxically, essential for maintaining the integrity of the intestinal barrier in a non-inflammatory state. However, the intracellular mechanisms that regulate distinct transcriptional profiles and functional diversity of Th17 cells remain unclear. Here we show Raftlin1, a lipid raft protein, specifically upregulates and forms a complex with RORγt in pathogenic Th17 cells. Disruption of the RORγt-Raftlin1 complex results in the reduction of pathogenic Th17 cells in response to Citrobacter rodentium; however, there is no effect on nonpathogenic Th17 cells in response to commensal segmented filamentous bacteria. Mechanistically, we show that Raftlin1 recruits distinct phospholipids to RORγt and promotes the pathogenicity of Th17 cells. Thus, we have identified a mechanism that drives the pathogenic function of Th17 cells, which could provide a platform for advanced therapeutic strategies to dampen Th17-mediated inflammatory diseases.
-
-
-
Immunology and Microbiology
Lysophosphatidylcholine facilitates the pathogenesis of psoriasis through activating keratinocytes and T cells differentiation via glycolysis.
In J Eur Acad Dermatol Venereol on 1 July 2023 by Liu, P., Zhou, Y., et al.
PubMed
Although abnormal metabolism plays a critical role in the pathogenesis of psoriasis, the details are unclear.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Immune mechanisms shape the clonal landscape during early progression of prostate cancer.
In Dev Cell on 19 June 2023 by Tshering, L. F., Luo, F., et al.
PubMed
Understanding the role of the immune microenvironment in modulating intratumor heterogeneity is essential for effective cancer therapies. Using multicolor lineage tracing in genetically engineered mouse models and single-cell transcriptomics, we show that slowly progressing tumors contain a multiclonal landscape of relatively homogeneous subpopulations within a well-organized tumor microenvironment. In more advanced and aggressive tumors, however, the multiclonal landscape develops into competing dominant and minor clones accompanied by a disordered microenvironment. We demonstrate that this dominant/minor landscape is associated with differential immunoediting, in which minor clones are marked by an increased expression of IFNγ-response genes and the T cell-activating chemokines Cxcl9 and Cxcl11. Furthermore, immunomodulation of the IFNγ pathway can rescue minor clones from elimination. Notably, the immune-specific gene signature of minor clones exhibits a prognostic value for biochemical recurrence-free survival in human prostate cancer. These findings suggest new immunotherapy approaches for modulating clonal fitness and tumor progression in prostate cancer.
-
-
-
Flow cytometry/Cell sorting
-
Cancer Research
-
Immunology and Microbiology
Enhancement of T cell infiltration via tumor-targeted Th9 cell delivery improves the efficacy of antitumor immunotherapy of solid tumors.
In Bioact Mater on 1 May 2023 by Chen, T., Xue, Y., et al.
PubMed
Insufficient infiltration of T cells severely compromises the antitumor efficacy of adoptive cell therapy (ACT) against solid tumors. Here, we present a facile immune cell surface engineering strategy aiming to substantially enhance the anti-tumor efficacy of Th9-mediated ACT by rapidly identifying tumor-specific binding ligands and improving the infiltration of infused cells into solid tumors. Non-genetic decoration of Th9 cells with tumor-targeting peptide screened from phage display not only allowed precise targeted ACT against highly heterogeneous solid tumors but also substantially enhanced infiltration of CD8+ T cells, which led to improved antitumor outcomes. Mechanistically, infusion of Th9 cells modified with tumor-specific binding ligands facilitated the enhanced distribution of tumor-killing cells and remodeled the immunosuppressive microenvironment of solid tumors via IL-9 mediated immunomodulation. Overall, we presented a simple, cost-effective, and cell-friendly strategy to enhance the efficacy of ACT against solid tumors with the potential to complement the current ACT.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Lipidomic profiling reveals metabolic signatures in psoriatic skin lesions.
In Clin Immunol on 1 January 2023 by Liu, P., Hou, G., et al.
PubMed
Psoriasis is a chronic immune-mediated inflammatory disease. Lipids play an important role in regulating the inflammatory response. However, the alteration of lipids involved in psoriasis particular in skin lesions remain unclear. Here, we performed the lipidomics to investigate lipid profiling in the skin lesions of the imiquimod-induced psoriasis-like dermatitis and psoriasis patients. The findings showed that ceramides phosphate (CerP) and ceramides were enriched in psoriatic lesions compared with controls from both psoriasis patients and psoriasis-like mouse model. Psoriasis patients were classified into two subtypes, the CC1 and CC2, by consensus clustering of these lipid signatures. The CC1 was characterized by the higher levels of CerP, uric acid, and more severe psoriasis, compared with CC2 subtype. Interestingly, ceramide-1-phosphate (C1P), dramatically enriched in CC1 subtype, facilitated imiquimod-induced psoriasis-like inflammatory responses. Mechanistically, C1P induced the expression of inflammatory factors and activated DNA replication and cell cycle signaling pathways in the primary keratinocytes. Inhibiting the production of C1P with ceramide kinase inhibitor effectively alleviated the imiquimod-induced psoriasis-like inflammation. Taken together, we described the landscape of lipids alteration and established lipids classification based on pattern of abundance of lipids in psoriatic skin lesions. Suppression of C1P pathway is a novel potential strategy for psoriasis treatment.
-
-
-
Immunology and Microbiology
Pak2-mediated phosphorylation promotes RORγt ubiquitination and inhibits colonic inflammation.
In Cell Rep on 13 September 2022 by Kathania, M., Kumar, R., et al.
PubMed
Dysregulated interleukin-17 (IL-17) expression and its downstream signaling is strongly linked to inflammatory bowel diseases (IBDs). However, the molecular mechanisms by which the function of RORγt, the transcription factor of IL-17, is regulated remains elusive. By a mass spectrometry-based approach, we identify that Pak2, a serine (S)/threonine (T) kinase, directly associates with RORγt. Pak2 recognizes a conserved KRLS motif within RORγt and phosphorylates the S-316 within this motif. Genetic deletion of Pak2 in Th17 cells reduces RORγt phosphorylation, increases IL-17 expression, and induces severe colitis upon adoptive transfer to Rag1-/- mice. Similarly, reconstitution of RORγt-S316A mutant in Rorc-/- Th17 cells enhances IL-17 expression and colitis severity. Mechanistically, we demonstrate that Pak2-mediated phosphorylation causes a conformational change resulting in exposure of the ubiquitin ligase Itch interacting PPLY motif and degradation of RORγt. Thus, we have uncovered a mechanism by which the activity of RORγt is regulated that can be exploited therapeutically.
-
-
-
Immunology and Microbiology
Evaluation of Glutaminolysis in T Cells.
In Curr Protoc on 1 September 2022 by Tajima, M., Strober, W., et al.
PubMed
The activity of living cells is necessarily dependent on the amount of available bioenergy. In T cells, the latter is mainly derived from ATP, a molecular energy "coin" generated by one of several metabolic processes that differ in their ability to satisfy energy demand. Thus, whereas naïve or quiescent T cells efficiently utilize oxidative phosphorylation to generate ATP, T cells subjected to antigenic stimulation followed by clonal expansion and cytokine production meet their increased need for energy by supplementing ATP generation by oxidative phosphorylation with ATP generation by glycolysis. Yet additional need for ATP can be met by other basic biologic sources of energy such as glutamine, an amino acid that is metabolized through a process called glutaminolysis to result in end products that flows into the TCA cycle and augment ATP generation by oxidative phosphorylation. It is now possible to track the dominant energy supplying processes (i.e., the ATP generation process) in differentiating or activated T cells in a real-time manner. Here, we provide one element of such tracking by describing protocols for the assessment of the contribution of glutaminolysis to overall ATP production within different T cell subsets. © 2022 Wiley Periodicals LLC. This article has been contributed to by US Government employees and their work is in the public domain in the USA. Basic Protocol 1: Evaluation of the role of glutaminolysis during T cell activation/differentiation Basic Protocol 2: Evaluation of the role of glutaminolysis in T cell responses utilizing glutaminolysis inhibitors Basic Protocol 3: Evaluation of the effect of glutaminolysis on cellular oxidative phosphorylation/glycolysis.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
Lipidomic profiling reveals metabolic signatures in psoriatic skin lesions
In Research Square on 27 July 2022 by Peng, C., Liu, P., et al.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
Re-programming mouse liver-resident invariant natural killer T cells for suppressing hepatic and diabetogenic autoimmunity.
In Nat Commun on 7 June 2022 by Umeshappa, C. S., Solé, P., et al.
PubMed
Invariant NKT (iNKT) cells comprise a heterogeneous group of non-circulating, tissue-resident T lymphocytes that recognize glycolipids, including alpha-galactosylceramide (αGalCer), in the context of CD1d, but whether peripheral iNKT cell subsets are terminally differentiated remains unclear. Here we show that mouse and human liver-resident αGalCer/CD1d-binding iNKTs largely correspond to a novel Zbtb16+Tbx21+Gata3+MaflowRorc- subset that exhibits profound transcriptional, phenotypic and functional plasticity. Repetitive in vivo encounters of these liver iNKT (LiNKT) cells with intravenously delivered αGalCer/CD1d-coated nanoparticles (NP) trigger their differentiation into immunoregulatory, IL-10+IL-21-producing Zbtb16highMafhighTbx21+Gata3+Rorc- cells, termed LiNKTR1, expressing a T regulatory type 1 (TR1)-like transcriptional signature. This response is LiNKT-specific, since neither lung nor splenic tissue-resident iNKT cells from αGalCer/CD1d-NP-treated mice produce IL-10 or IL-21. Additionally, these LiNKTR1 cells suppress autoantigen presentation, and recognize CD1d expressed on conventional B cells to induce IL-10+IL-35-producing regulatory B (Breg) cells, leading to the suppression of liver and pancreas autoimmunity. Our results thus suggest that LiNKT cells are plastic for further functional diversification, with such plasticity potentially targetable for suppressing tissue-specific inflammatory phenomena.
-
-
-
In vivo experiments
-
Immunology and Microbiology
Commensal Cryptosporidium colonization elicits a cDC1-dependent Th1 response that promotes intestinal homeostasis and limits other infections.
In Immunity on 9 November 2021 by Russler-Germain, E. V., Jung, J., et al.
PubMed
Cryptosporidium can cause severe diarrhea and morbidity, but many infections are asymptomatic. Here, we studied the immune response to a commensal strain of Cryptosporidium tyzzeri (Ct-STL) serendipitously discovered when conventional type 1 dendritic cell (cDC1)-deficient mice developed cryptosporidiosis. Ct-STL was vertically transmitted without negative health effects in wild-type mice. Yet, Ct-STL provoked profound changes in the intestinal immune system, including induction of an IFN-γ-producing Th1 response. TCR sequencing coupled with in vitro and in vivo analysis of common Th1 TCRs revealed that Ct-STL elicited a dominant antigen-specific Th1 response. In contrast, deficiency in cDC1s skewed the Ct-STL CD4 T cell response toward Th17 and regulatory T cells. Although Ct-STL predominantly colonized the small intestine, colon Th1 responses were enhanced and associated with protection against Citrobacter rodentium infection and exacerbation of dextran sodium sulfate and anti-IL10R-triggered colitis. Thus, Ct-STL represents a commensal pathobiont that elicits Th1-mediated intestinal homeostasis that may reflect asymptomatic human Cryptosporidium infection.
-
-
-
Cancer Research
Immunogenic camptothesome nanovesicles comprising sphingomyelin-derived camptothecin bilayers for safe and synergistic cancer immunochemotherapy.
In Nat Nanotechnol on 1 October 2021 by Wang, Z., Little, N., et al.
PubMed
Despite the enormous therapeutic potential of immune checkpoint blockade (ICB), it benefits only a small subset of patients. Some chemotherapeutics can switch 'immune-cold' tumours to 'immune-hot' to synergize with ICB. However, safe and universal therapeutic platforms implementing such immune effects remain scarce. We demonstrate that sphingomyelin-derived camptothecin nanovesicles (camptothesomes) elicit potent granzyme-B- and perforin-mediated cytotoxic T lymphocyte (CTL) responses, potentiating PD-L1/PD-1 co-blockade to eradicate subcutaneous MC38 adenocarcinoma with developed memory immunity. In addition, camptothesomes improve the pharmacokinetics and lactone stability of camptothecin, avoid systemic toxicities, penetrate deeply into the tumour and outperform the antitumour efficacy of Onivyde. Camptothesome co-load the indoleamine 2,3-dioxygenase inhibitor indoximod into its interior using the lipid-bilayer-crossing capability of the immunogenic cell death inducer doxorubicin, eliminating clinically relevant advanced orthotopic CT26-Luc tumours and late-stage B16-F10-Luc2 melanoma, and achieving complete metastasis remission when combined with ICB and folate targeting. The sphingomyelin-derived nanotherapeutic platform and doxorubicin-enabled transmembrane transporting technology are generalizable to various therapeutics, paving the way for transformation of the cancer immunochemotherapy paradigm.
-
-
-
In vivo experiments
-
Immunology and Microbiology
Epithelium-autonomous NAIP/NLRC4 prevents TNF-driven inflammatory destruction of the gut epithelial barrier in Salmonella-infected mice.
In Mucosal Immunol on 1 May 2021 by Fattinger, S. A., Geiser, P., et al.
PubMed
The gut epithelium is a critical protective barrier. Its NAIP/NLRC4 inflammasome senses infection by Gram-negative bacteria, including Salmonella Typhimurium (S.Tm) and promotes expulsion of infected enterocytes. During the first ~12-24 h, this reduces mucosal S.Tm loads at the price of moderate enteropathy. It remained unknown how this NAIP/NLRC4-dependent tradeoff would develop during subsequent infection stages. In NAIP/NLRC4-deficient mice, S.Tm elicited severe enteropathy within 72 h, characterized by elevated mucosal TNF (>20 pg/mg) production from bone marrow-derived cells, reduced regeneration, excessive enterocyte loss, and a collapse of the epithelial barrier. TNF-depleting antibodies prevented this destructive pathology. In hosts proficient for epithelial NAIP/NLRC4, a heterogeneous enterocyte death response with both apoptotic and pyroptotic features kept S.Tm loads persistently in check, thereby preventing this dire outcome altogether. Our results demonstrate that immediate and selective removal of infected enterocytes, by locally acting epithelium-autonomous NAIP/NLRC4, is required to avoid a TNF-driven inflammatory hyper-reaction that otherwise destroys the epithelial barrier.
-
-
-
In vivo experiments
-
Immunology and Microbiology
Niche-specific MHC II and PD-L1 regulate CD4+CD8αα+ intraepithelial lymphocyte differentiation.
In J Exp Med on 5 April 2021 by Moon, S., Park, Y., et al.
PubMed
Conventional CD4+ T cells are differentiated into CD4+CD8αα+ intraepithelial lymphocytes (IELs) in the intestine; however, the roles of intestinal epithelial cells (IECs) are poorly understood. Here, we showed that IECs expressed MHC class II (MHC II) and programmed death-ligand 1 (PD-L1) induced by the microbiota and IFN-γ in the distal part of the small intestine, where CD4+ T cells were transformed into CD4+CD8αα+ IELs. Therefore, IEC-specific deletion of MHC II and PD-L1 hindered the development of CD4+CD8αα+ IELs. Intracellularly, PD-1 signals supported the acquisition of CD8αα by down-regulating the CD4-lineage transcription factor, T helper-inducing POZ/Krüppel-like factor (ThPOK), via the Src homology 2 domain-containing tyrosine phosphatase (SHP) pathway. Our results demonstrate that noncanonical antigen presentation with cosignals from IECs constitutes niche adaptation signals to develop tissue-resident CD4+CD8αα+ IELs.
-