InVivoMAb anti-mouse/human VLA-4 (CD49d)
Product Description
Specifications
| Isotype | Rat IgG2b, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin |
| Recommended Dilution Buffer | InVivoPure pH 6.5 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse P815 mast cells |
| Reported Applications |
in vivo VLA-4 neutralization in vitro VLA-4 neutralization Flow cytometry |
| Formulation |
PBS, pH 6.5 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107657 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo VLA-4 neutralization
Badell, I. R., et al (2015). "Pathogen Stimulation History Impacts Donor-Specific CD8 T Cell Susceptibility to Costimulation/Integrin Blockade-Based Therapy" Am J Transplant. doi : 10.1111/ajt.13399.
PubMed
Recent studies have shown that the quantity of donor-reactive memory T cells is an important factor in determining the relative heterologous immunity barrier posed during transplantation. Here, we hypothesized that the quality of T cell memory also potently influences the response to costimulation blockade-based immunosuppression. Using a murine skin graft model of CD8+ memory T cell-mediated costimulation blockade resistance, we elicited donor-reactive memory T cells using three distinct types of pathogen infections. Strikingly, we observed differential efficacy of a costimulation and integrin blockade regimen based on the type of pathogen used to elicit the donor-reactive memory T cell response. Intriguingly, the most immunosuppression-sensitive memory T cell populations were composed primarily of central memory cells that possessed greater recall potential, exhibited a less differentiated phenotype, and contained more multi-cytokine producers. These data, therefore, demonstrate that the memory T cell barrier is dependent on the specific type of pathogen infection via which the donor-reactive memory T cells are elicited, and suggest that the immune stimulation history of a given transplant patient may profoundly influence the relative barrier posed by heterologous immunity during transplantation.
in vivo VLA-4 neutralization
Guidotti, L. G., et al (2015). "Immunosurveillance of the liver by intravascular effector CD8(+) T cells" Cell 161(3): 486-500.
PubMed
Effector CD8(+) T cells (CD8 TE) play a key role during hepatotropic viral infections. Here, we used advanced imaging in mouse models of hepatitis B virus (HBV) pathogenesis to understand the mechanisms whereby these cells home to the liver, recognize antigens, and deploy effector functions. We show that circulating CD8 TE arrest within liver sinusoids by docking onto platelets previously adhered to sinusoidal hyaluronan via CD44. After the initial arrest, CD8 TE actively crawl along liver sinusoids and probe sub-sinusoidal hepatocytes for the presence of antigens by extending cytoplasmic protrusions through endothelial fenestrae. Hepatocellular antigen recognition triggers effector functions in a diapedesis-independent manner and is inhibited by the processes of sinusoidal defenestration and capillarization that characterize liver fibrosis. These findings reveal the dynamic behavior whereby CD8 TE control hepatotropic pathogens and suggest how liver fibrosis might reduce CD8 TE immune surveillance toward infected or transformed hepatocytes.
in vivo LFA-1 neutralization
Ren, W., et al (2015). "Surrogate light chain is required for central and peripheral B-cell tolerance and inhibits anti-DNA antibody production by marginal zone B cells" Eur J Immunol 45(4): 1228-1237.
PubMed
Selection of the primary antibody repertoire takes place in pro-/pre-B cells, and subsequently in immature and transitional B cells. At the first checkpoint, mu heavy (muH) chains assemble with surrogate light (SL) chain into a precursor B-cell receptor. In mice lacking SL chain, muH chain selection is impaired, and serum autoantibody levels are elevated. However, whether the development of autoantibody-producing cells is due to an inability of the resultant B-cell receptors to induce central and/or peripheral B-cell tolerance or other factors is unknown. Here, we show that receptor editing is defective, and that a higher proportion of BM immature B cells are prone to undergoing apoptosis. Furthermore, transitional B cells are also more prone to undergoing apoptosis, with a stronger selection pressure to enter the follicular B-cell pool. Those that enter the marginal zone (MZ) B-cell pool escape selection and survive, possibly due to the B-lymphopenia and elevated levels of B-cell activating factor. Moreover, the MZ B cells are responsible for the elevated IgM anti-dsDNA antibody levels detected in these mice. Thus, the SL chain is required for central and peripheral B-cell tolerance and inhibits anti-DNA antibody production by MZ B cells.
in vivo VLA-4 neutralization
Flow Cytometry
Wang, X., et al (2014). "Integrin-mediated interactions between B cells and follicular dendritic cells influence germinal center B cell fitness" J Immunol 192(10): 4601-4609.
PubMed
Integrin-ligand interactions between germinal center (GC) B cells and Ag-presenting follicular dendritic cells (FDCs) have been suggested to play central roles during GC responses, but their in vivo requirement has not been directly tested. In this study, we show that, whereas integrins alphaLbeta2 and alpha4beta1 are highly expressed and functional on mouse GC B cells, removal of single integrins or their ligands had little effect on B cell participation in the GC response. Combined beta2 integrin deficiency and alpha4 integrin blockade also did not affect the GC response against a particulate Ag. However, the combined integrin deficiency did cause B cells to be outcompeted in splenic GC responses against a soluble protein Ag and in mesenteric lymph node GC responses against gut-derived Ags. Similar findings were made for beta2-deficient B cells in mice lacking VCAM1 on FDCs. The reduced fitness of the GC B cells did not appear to be due to decreased Ag acquisition, proliferation rates, or pAKT levels. In summary, our findings provide evidence that alphaLbeta2 and alpha4beta1 play overlapping and context-dependent roles in supporting interactions with FDCs that can augment the fitness of responding GC B cells. We also find that mouse GC B cells upregulate alphavbeta3 and adhere to vitronectin and milk-fat globule epidermal growth factor VIII protein. Integrin beta3-deficient B cells contributed in a slightly exaggerated manner to GC responses, suggesting this integrin has a regulatory function in GC B cells.
in vitro VLA-4 neutralization
Schmidt, T. H., et al (2013). "CXCR4 promotes B cell egress from Peyer’s patches" J Exp Med 210(6): 1099-1107.
PubMed
Peyer’s patches (PPs) play a central role in supporting B cell responses against intestinal antigens, yet the factors controlling B cell passage through these mucosal lymphoid tissues are incompletely understood. We report that, in mixed chimeras, CXCR4-deficient B cells accumulate in PPs compared with their representation in other lymphoid tissues. CXCR4-deficient B cells egress from PPs more slowly than wild-type cells, whereas CXCR5-deficient cells egress more rapidly. The CXCR4 ligand, CXCL12, is expressed by cells adjacent to lymphatic endothelial cells in a zone that abuts but minimally overlaps with the CXCL13(+) follicle. CXCR4-deficient B cells show reduced localization to these CXCL12(+) perilymphatic zones, whereas CXCR5-deficient B cells preferentially localize in these regions. By photoconverting KikGR-expressing cells within surgically exposed PPs, we provide evidence that naive B cells transit PPs with an approximate residency half-life of 10 h. When CXCR4 is lacking, KikGR(+) B cells show a delay in PP egress. In summary, we identify a CXCL12(hi) perilymphatic zone in PPs that plays a role in overcoming CXCL13-mediated retention to promote B cell egress from these gut-associated lymphoid tissues.
in vivo VLA-4 neutralization
in vitro VLA-4 neutralization
Walch, J. M., et al (2013). "Cognate antigen directs CD8+ T cell migration to vascularized transplants" J Clin Invest 123(6): 2663-2671.
PubMed
The migration of effector or memory T cells to the graft is a critical event in the rejection of transplanted organs. The prevailing view is that the key steps involved in T cell migration – integrin-mediated firm adhesion followed by transendothelial migration – are dependent on the activation of Galphai-coupled chemokine receptors on T cells. In contrast to this view, we demonstrated in vivo that cognate antigen was necessary for the firm adhesion and transendothelial migration of CD8+ effector T cells specific to graft antigens and that both steps occurred independent of Galphai signaling. Presentation of cognate antigen by either graft endothelial cells or bone marrow-derived APCs that extend into the capillary lumen was sufficient for T cell migration. The adhesion and transmigration of antigen-nonspecific (bystander) effector T cells, on the other hand, remained dependent on Galphai, but required the presence of antigen-specific effector T cells. These findings underscore the primary role of cognate antigen presented by either endothelial cells or bone marrow-derived APCs in the migration of T cells across endothelial barriers and have important implications for the prevention and treatment of graft rejection.
in vivo VLA-4 neutralization
Kitchens, W. H., et al (2012). "Combined costimulatory and leukocyte functional antigen-1 blockade prevents transplant rejection mediated by heterologous immune memory alloresponses" Transplantation 93(10): 997-1005.
PubMed
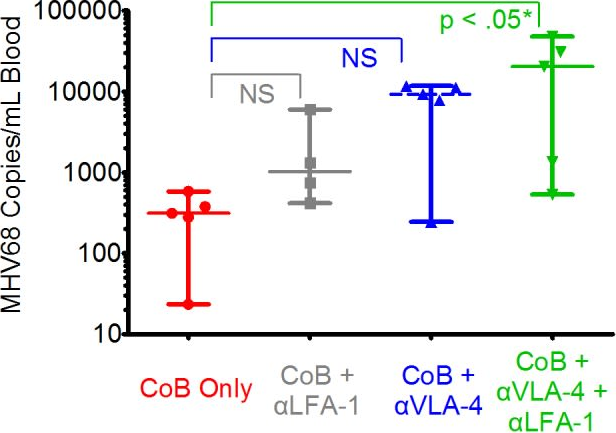
BACKGROUND: Recent evidence suggests that alloreactive memory T cells are generated by the process of heterologous immunity, whereby memory T cells arising in response to pathogen infection crossreact with donor antigens. Because of their diminished requirements for costimulation during recall, these pathogen-elicited allocrossreactive memory T cells are of particular clinical importance, especially given the emergence of costimulatory blockade as a transplant immunosuppression strategy. METHODS: We used an established model of heterologous immunity involving sequential infection of a naive C57BL/6 recipient with lymphocytic choriomeningitis virus and vaccinia virus, followed by combined skin and bone marrow transplant from a BALB/c donor. RESULTS: We demonstrate that coupling the integrin antagonist anti-leukocyte functional antigen (LFA)-1 with costimulatory blockade could surmount the barrier posed by heterologous immunity in a fully allogeneic murine transplant system. The combined costimulatory and integrin blockade regimen suppressed proliferation of alloreactive memory T cells and attenuated their cytokine effector responses. This combined blockade regimen also promoted the retention of FoxP(3)(+) Tregs in draining lymph nodes. Finally, we show that in an in vitro mixed lymphocyte reaction system using human T cells, the combination of belatacept and anti-LFA-1 was able to suppress cytokine production by alloreactive memory T cells that was resistant to belatacept alone. CONCLUSIONS: As an antagonist against human LFA-1 exists and has been used clinically to treat psoriasis, these findings have significant translational potential for future clinical transplant trials.
in vivo VLA-4 neutralization
in vitro VLA-4 neutralization
John, B., et al (2011). "Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis" PLoS Pathog 7(9): e1002246.
PubMed
Under normal conditions the immune system has limited access to the brain; however, during toxoplasmic encephalitis (TE), large numbers of T cells and APCs accumulate within this site. A combination of real time imaging, transgenic reporter mice, and recombinant parasites allowed a comprehensive analysis of CD11c+ cells during TE. These studies reveal that the CNS CD11c+ cells consist of a mixture of microglia and dendritic cells (DCs) with distinct behavior associated with their ability to interact with parasites or effector T cells. The CNS DCs upregulated several chemokine receptors during TE, but none of these individual receptors tested was required for migration of DCs into the brain. However, this process was pertussis toxin sensitive and dependent on the integrin LFA-1, suggesting that the synergistic effect of signaling through multiple chemokine receptors, possibly leading to changes in the affinity of LFA-1, is involved in the recruitment/retention of DCs to the CNS and thus provides new insights into how the immune system accesses this unique site.
in vivo VLA-4 neutralization
in vitro VLA-4 neutralization
Rothhammer, V., et al (2011). "Th17 lymphocytes traffic to the central nervous system independently of alpha4 integrin expression during EAE" J Exp Med 208(12): 2465-2476.
PubMed
The integrin alpha4beta1 (VLA-4) is used by encephalitogenic T cells to enter the central nervous system (CNS). However, both Th1 and Th17 cells are capable of inducing experimental autoimmune encephalomyelitis (EAE), and the molecular cues mediating the infiltration of Th1 versus Th17 cells into the CNS have not yet been defined. We investigated how blocking of alpha4 integrins affected trafficking of Th1 and Th17 cells into the CNS during EAE. Although antibody-mediated inhibition of alpha4 integrins prevented EAE when MOG(35-55)-specific Th1 cells were adoptively transferred, Th17 cells entered the brain, but not the spinal cord parenchyma, irrespective of alpha4 blockade. Accordingly, T cell-conditional alpha4-deficient mice were not resistant to actively induced EAE but showed an ataxic syndrome with predominantly supraspinal infiltrates of IL-23R(+)CCR6(+)CD4(+) T cells. The entry of alpha4-deficient Th17 cells into the CNS was abolished by blockade of LFA-1 (alphaLbeta2 integrin). Thus, Th1 cells preferentially infiltrate the spinal cord via an alpha4 integrin-mediated mechanism, whereas the entry of Th17 cells into the brain parenchyma occurs in the absence of alpha4 integrins but is dependent on the expression of alphaLbeta2. These observations have implications for the understanding of lesion localization, immunosurveillance, and drug design in multiple sclerosis.
in vivo VLA-4 neutralization
Thomas, S. Y., et al (2011). "PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions" J Exp Med 208(6): 1179-1188.
PubMed
Innate-like NKT cells conspicuously accumulate within the liver microvasculature of healthy mice, crawling on the luminal side of endothelial cells, but their general recirculation pattern and the mechanism of their intravascular behavior have not been elucidated. Using parabiotic mice, we demonstrated that, despite their intravascular location, most liver NKT cells failed to recirculate. Antibody blocking experiments established that they were retained locally through constitutive LFA-1-intercellular adhesion molecule (ICAM) 1 interactions. This unprecedented lifelong intravascular residence could be induced in conventional CD4 T cells by the sole expression of promyelocytic leukemia zinc finger (PLZF), a transcription factor specifically expressed in the NKT lineage. These findings reveal the unique genetic and biochemical pathway that underlies the innate intravascular surveillance program of NKT cells.
Product Citations
-
-
Immunology and Microbiology
-
Cancer Research
Double-positive T cells form heterotypic clusters with circulating tumor cells to foster cancer metastasis.
In J Clin Invest on 16 September 2025 by Scholten, D., El-Shennawy, L., et al.
PubMed
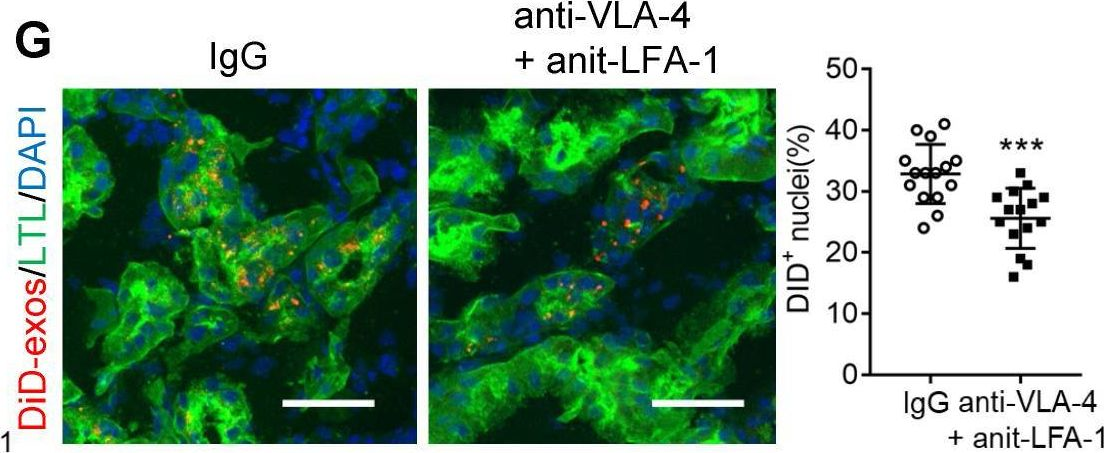
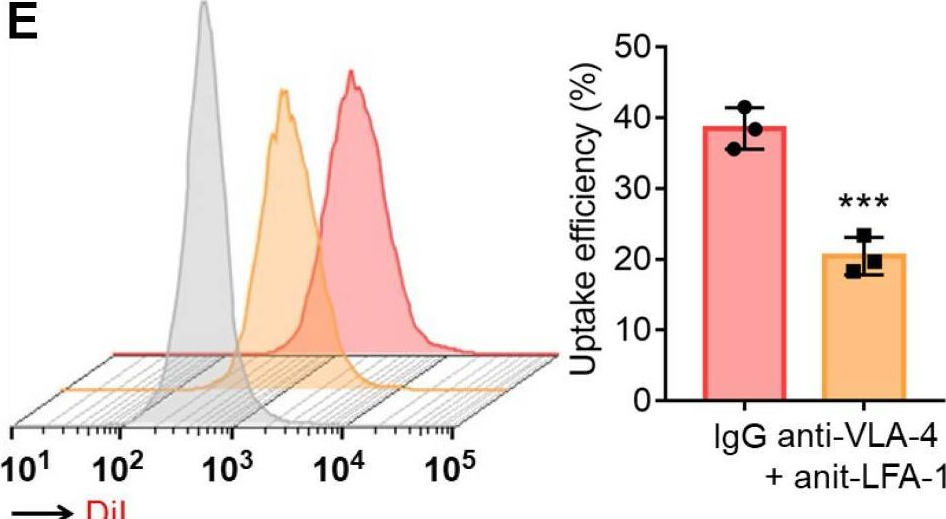
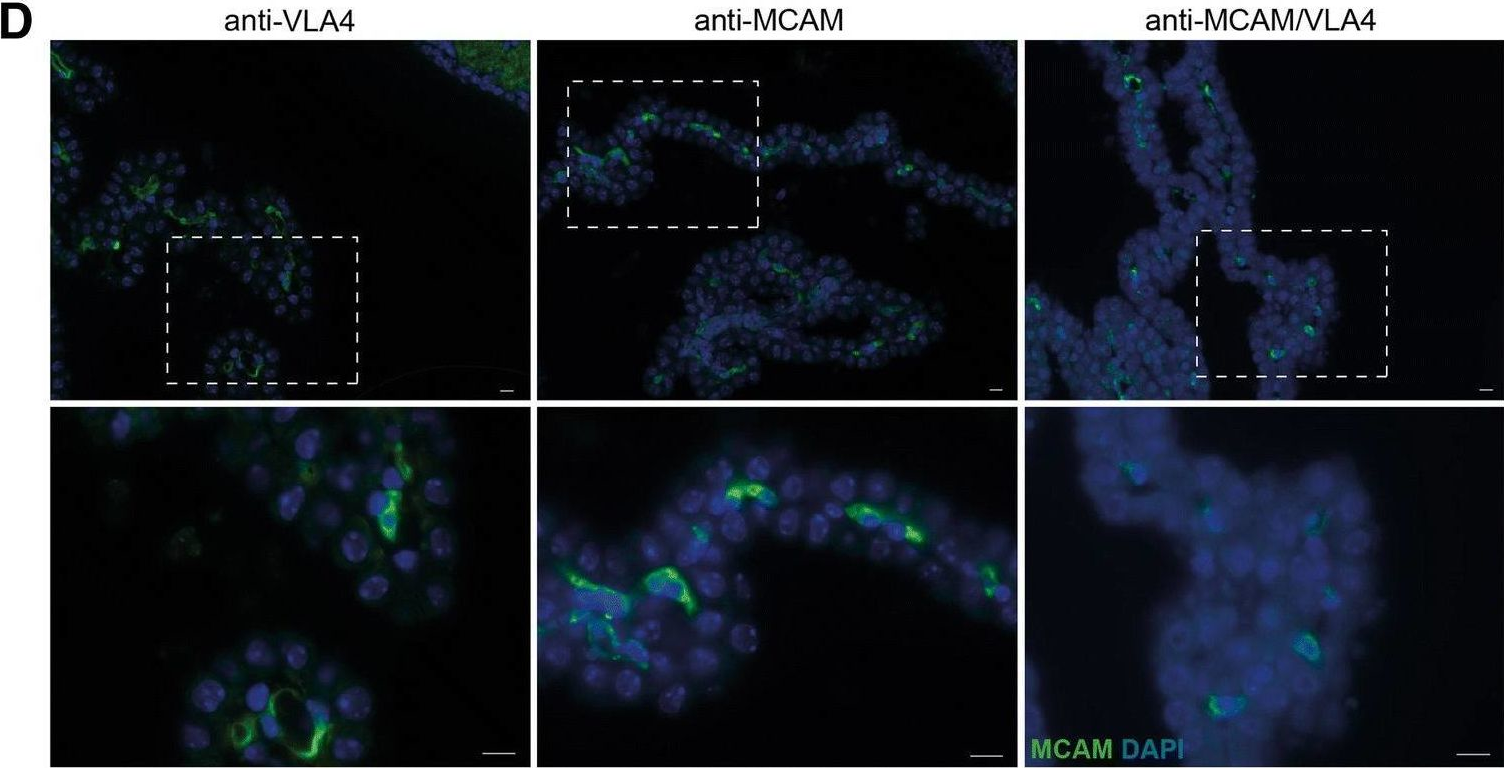
The immune ecosystem is central to maintaining effective defensive responses. However, it remains largely understudied how immune cells in the peripheral blood interact with circulating tumor cells (CTCs) in metastasis. Here, blood analysis of patients with advanced breast cancer revealed that over 75% of CTC-positive blood specimens contained heterotypic CTC clusters with CD45+ white blood cells (WBCs), which correlates with breast cancer subtypes, racial groups, and decreased survival. CTC-WBC clusters included overrepresented T cells and underrepresented neutrophils. Specifically, a rare subset of CD4 and CD8 double-positive T (DPT) cells was 140-fold enriched in CTC clusters versus their frequency in WBCs. DPT cells shared properties with CD4+ and CD8+ T cells but exhibited unique features of T cell exhaustion and immune suppression. Mechanistically, the integrin heterodimer α4β1, also named very late antigen 4 (VLA-4), in DPT cells and its ligand, VCAM1, in tumor cells are essential mediators of DPT-CTC clusters. Neoadjuvant administration of anti-VLA-4 neutralizing antibodies markedly blocked CTC-DPT clusters, inhibited metastasis, and extended mouse survival. These findings highlight a pivotal role of rare DPT cells in fostering cancer dissemination through CTC clustering. It lays a foundation for developing innovative biomarker-guided therapeutic strategies to prevent and target cancer metastasis.
-
-
FcRn-silencing of IL-12Fc prevents toxicity of local IL-12 therapy and prolongs survival in experimental glioblastoma.
In Nat Commun on 22 May 2025 by Beffinger, M. M., Schellhammer, L., et al.
PubMed
Glioblastoma remains a challenging indication for immunotherapy: the blood-brain barrier hampers accessibility for systemic treatments and the immunosuppressive microenvironment impedes immune attack. Intratumoral therapy with the proinflammatory cytokine interleukin-12 (IL-12) can revert immunosuppression but leakage into the circulation causes treatment-limiting toxicity. Here we engineer an IL-12Fc fusion cytokine with reduced binding to the neonatal Fc receptor FcRn. FcRn-silenced IL-12Fc avoids FcRn-mediated brain export, thus exhibits prolonged brain retention and reduced blood levels, which prevents toxicity. In murine glioblastoma, FcRn-silenced IL-12Fc induces more durable responses with negligible systemic cytokine exposure and boosts the efficacy of radio- and chemotherapy. It triggers anti-tumor responses independently of peripheral T cell influx or lymphopenia and leads to inflammatory polarization of the tumor microenvironment in patient-derived glioblastoma explants. FcRn-silencing of IL-12Fc may unlock the full potential of IL-12 for brain cancer therapy and could be further applied to containing the activity of other therapeutics targeting neurological diseases.
-
-
Immunology and Microbiology
TSP-1-CD47-integrin α4β1 axis drives T cell infiltration and synovial inflammation in rheumatoid arthritis.
In Front Immunol on 1 May 2025 by Hu, J., Wang, X., et al.
PubMed
Immune cell infiltration into joint synovial tissue and promotion of the inflammatory response are important processes in rheumatoid arthritis (RA). This article delves into the crucial role of CD47 in these processes, as well as the mechanisms at both cellular and molecular levels.
-
-
-
Cancer Research
-
Immunology and Microbiology
Rare Subset of T Cells Form Heterotypic Clusters with Circulating Tumor Cells to Foster Cancer Metastasis
In bioRxiv on 3 April 2025 by Scholten, D., El-Shennawy, L., et al.
-
-
-
Immunology and Microbiology
Inflammation switches the chemoattractant requirements for naive lymphocyte entry into lymph nodes.
In Cell on 20 February 2025 by Chen, K. Y., De Giovanni, M., et al.
PubMed
Sustained lymphocyte migration from blood into lymph nodes (LNs) is important for immune responses. The CC-chemokine receptor-7 (CCR7) ligand CCL21 is required for LN entry but is downregulated during inflammation, and it has been unclear how recruitment is maintained. Here, we show that the oxysterol biosynthetic enzyme cholesterol-25-hydroxylase (Ch25h) is upregulated in LN high endothelial venules during viral infection. Lymphocytes become dependent on oxysterols, generated through a transcellular endothelial-fibroblast metabolic pathway, and the receptor EBI2 for inflamed LN entry. Additionally, Langerhans cells are an oxysterol source. Ch25h is also expressed in inflamed peripheral endothelium, and EBI2 mediates B cell recruitment in a tumor model. Finally, we demonstrate that LN CCL19 is critical in lymphocyte recruitment during inflammation. Thus, our work explains how naive precursor trafficking is sustained in responding LNs, identifies a role for oxysterols in cell recruitment into inflamed tissues, and establishes a logic for the CCR7 two-ligand system.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Anti-CD49d Ab treatment ameliorates age-associated inflammatory response and mitigates CD8+ T-cell cytotoxicity after traumatic brain injury.
In J Neuroinflammation on 19 October 2024 by Chen, Z., Ford, K. P., et al.
PubMed
Patients aged 65 years and older account for an increasing proportion of patients with traumatic brain injury (TBI). Older TBI patients experience increased morbidity and mortality compared to their younger counterparts. Our prior data demonstrated that by blocking α4 integrin, anti-CD49d antibody (aCD49d Ab) abrogates CD8+ T-cell infiltration into the injured brain, improves survival, and attenuates neurocognitive deficits. Here, we aimed to uncover how aCD49d Ab treatment alters local cellular responses in the aged mouse brain. Consequently, mice incur age-associated toxic cytokine and chemokine responses long-term post-TBI. aCD49d Ab attenuates this response along with a T helper (Th)1/Th17 immunological shift and remediation of overall CD8+ T cell cytotoxicity. Furthermore, aCD49d Ab reduces CD8+ T cells exhibiting higher effector status, leading to reduced clonal expansion in aged, but not young, mouse brains with chronic TBI. Together, aCD49d Ab is a promising therapeutic strategy for treating TBI in the older people.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
antiCD49d Ab treatment ameliorates age-associated inflammatory response and mitigates CD8+ T-cell cytotoxicity after traumatic brain injury
In bioRxiv on 22 June 2024 by Chen, Z., Ford, K. P., et al.
-
-
-
Genetics
-
Immunology and Microbiology
Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma.
In Immunity on 14 May 2024 by De Leo, A., Ugolini, A., et al.
PubMed
Immunosuppressive macrophages restrict anti-cancer immunity in glioblastoma (GBM). Here, we studied the contribution of microglia (MGs) and monocyte-derived macrophages (MDMs) to immunosuppression and mechanisms underlying their regulatory function. MDMs outnumbered MGs at late tumor stages and suppressed T cell activity. Molecular and functional analysis identified a population of glycolytic MDM expressing GLUT1 with potent immunosuppressive activity. GBM-derived factors promoted high glycolysis, lactate, and interleukin-10 (IL-10) production in MDMs. Inhibition of glycolysis or lactate production in MDMs impaired IL-10 expression and T cell suppression. Mechanistically, intracellular lactate-driven histone lactylation promoted IL-10 expression, which was required to suppress T cell activity. GLUT1 expression on MDMs was induced downstream of tumor-derived factors that activated the PERK-ATF4 axis. PERK deletion in MDM abrogated histone lactylation, led to the accumulation of intratumoral T cells and tumor growth delay, and, in combination with immunotherapy, blocked GBM progression. Thus, PERK-driven glucose metabolism promotes MDM immunosuppressive activity via histone lactylation.
-
-
-
Cancer Research
IL-7 mediated upregulation of VLA-4 increases accumulation of adoptively transferred T lymphocytes in murine glioma.
In bioRxiv on 1 April 2024 by Singh, K., Hotchkiss, K. M., et al.
-
-
-
Immunology and Microbiology
-
Cancer Research
Motility and tumor infiltration are key aspects of invariant natural killer T cell anti-tumor function.
In Nat Commun on 9 February 2024 by Tian, C., Wang, Y., et al.
PubMed
Dysfunction of invariant natural killer T (iNKT) cells contributes to immune resistance of tumors. Most mechanistic studies focus on their static functional status before or after activation, not considering motility as an important characteristic for antigen scanning and thus anti-tumor capability. Here we show via intravital imaging, that impaired motility of iNKT cells and their exclusion from tumors both contribute to the diminished anti-tumor iNKT cell response. Mechanistically, CD1d, expressed on macrophages, interferes with tumor infiltration of iNKT cells and iNKT-DC interactions but does not influence their intratumoral motility. VCAM1, expressed by cancer cells, restricts iNKT cell motility and inhibits their antigen scanning and activation by DCs via reducing CDC42 expression. Blocking VCAM1-CD49d signaling improves motility and activation of intratumoral iNKT cells, and consequently augments their anti-tumor function. Interference with macrophage-iNKT cell interactions further enhances the anti-tumor capability of iNKT cells. Thus, our findings provide a direction to enhance the efficacy of iNKT cell-based immunotherapy via motility regulation.
-
-
-
Cancer Research
The local microenvironment drives activation of neutrophils in human brain tumors.
In Cell on 12 October 2023 by Maas, R. R., Soukup, K., et al.
PubMed
Neutrophils are abundant immune cells in the circulation and frequently infiltrate tumors in substantial numbers. However, their precise functions in different cancer types remain incompletely understood, including in the brain microenvironment. We therefore investigated neutrophils in tumor tissue of glioma and brain metastasis patients, with matched peripheral blood, and herein describe the first in-depth analysis of neutrophil phenotypes and functions in these tissues. Orthogonal profiling strategies in humans and mice revealed that brain tumor-associated neutrophils (TANs) differ significantly from blood neutrophils and have a prolonged lifespan and immune-suppressive and pro-angiogenic capacity. TANs exhibit a distinct inflammatory signature, driven by a combination of soluble inflammatory mediators including tumor necrosis factor alpha (TNF-ɑ) and Ceruloplasmin, which is more pronounced in TANs from brain metastasis versus glioma. Myeloid cells, including tumor-associated macrophages, emerge at the core of this network of pro-inflammatory mediators, supporting the concept of a critical myeloid niche regulating overall immune suppression in human brain tumors.
-
-
-
Cancer Research
-
Immunology and Microbiology
Tumor-derived GDF-15 blocks LFA-1 dependent T cell recruitment and suppresses responses to anti-PD-1 treatment.
In Nat Commun on 20 July 2023 by Haake, M., Haack, B., et al.
PubMed
Immune checkpoint blockade therapy is beneficial and even curative for some cancer patients. However, the majority don't respond to immune therapy. Across different tumor types, pre-existing T cell infiltrates predict response to checkpoint-based immunotherapy. Based on in vitro pharmacological studies, mouse models and analyses of human melanoma patients, we show that the cytokine GDF-15 impairs LFA-1/β2-integrin-mediated adhesion of T cells to activated endothelial cells, which is a pre-requisite of T cell extravasation. In melanoma patients, GDF-15 serum levels strongly correlate with failure of PD-1-based immune checkpoint blockade therapy. Neutralization of GDF-15 improves both T cell trafficking and therapy efficiency in murine tumor models. Thus GDF-15, beside its known role in cancer-related anorexia and cachexia, emerges as a regulator of T cell extravasation into the tumor microenvironment, which provides an even stronger rationale for therapeutic anti-GDF-15 antibody development.
-
-
-
Neuroscience
Natural killer cells and innate lymphoid cells 1 tune anxiety-like behavior and memory in mice via interferon-γ and acetylcholine.
In Nat Commun on 29 May 2023 by Garofalo, S., Cocozza, G., et al.
PubMed
The mechanisms of communication between the brain and the immune cells are still largely unclear. Here, we characterize the populations of resident natural killer (NK) cells and innate lymphoid cells (ILC) 1 in the meningeal dura layer of adult mice. We describe that ILC1/NK cell-derived interferon-γ and acetylcholine can contribute to the modulation of brain homeostatic functions, shaping synaptic neuronal transmission and neurotransmitter levels with effects on mice behavior. In detail, the interferon-γ plays a role in the formation of non-spatial memory, tuning the frequency of GABAergic neurotransmission on cortical pyramidal neurons, while the acetylcholine is a mediator involved in the modulation of brain circuitries that regulate anxiety-like behavior. These findings disclose mechanisms of immune-to-brain communication that modulate brain functions under physiological conditions.
-
-
Three-dimensional morphologic and molecular atlases of nasal vasculature.
In Nat Cardiovasc Res on 1 May 2023 by Hong, S. P., Yang, M. J., et al.
PubMed
Understanding the function of the nasal vasculature in homeostasis and pathogenesis of common nasal diseases is important. Here we describe an extensive network of venous sinusoids (VSs) in mouse and human nasal mucosa. The endothelium of the VSs expressed Prox1 (considered to be a constitutive marker of lymphatic endothelium) and high levels of VCAM-1 and exhibited unusual cell-to-cell junctions. VSs are supported by circular smooth muscle cells (SMCs) and surrounded by immune cells. The nasal mucosa also showed a rich supply of lymphatic vessels with distinctive features, such as the absence of the lymphatic marker LYVE1 and sharp-ended capillaries. In mouse models of allergic rhinitis or acute Coronavirus Disease 2019 (COVID-19) infection, Prox1+ VSs were regressed or compromised. However, in aged mice, the VSs lost the SMC support and were expanded and enlarged. Our findings demonstrate three-dimensional morphological and molecular heterogeneities of the nasal vasculature and offer insights into their associations with nasal inflammation, infection and aging.
-
-
Immunology and Microbiology
Influence of circadian clocks on adaptive immunity and vaccination responses.
In Nat Commun on 30 January 2023 by Ince, L., Barnoud, C., et al.
PubMed
The adaptive immune response is under circadian control, yet, why adaptive immune reactions continue to exhibit circadian changes over long periods of time is unknown. Using a combination of experimental and mathematical modeling approaches, we show here that dendritic cells migrate from the skin to the draining lymph node in a time-of-day-dependent manner, which provides an enhanced likelihood for functional interactions with T cells. Rhythmic expression of TNF in the draining lymph node enhances BMAL1-controlled ICAM-1 expression in high endothelial venules, resulting in lymphocyte infiltration and lymph node expansion. Lymph node cellularity continues to be different for weeks after the initial time-of-day-dependent challenge, which governs the immune response to vaccinations directed against Hepatitis A virus as well as SARS-CoV-2. In this work, we present a mechanistic understanding of the time-of-day dependent development and maintenance of an adaptive immune response, providing a strategy for using time-of-day to optimize vaccination regimes.
-
-
-
Enzyme-linked immunosorbent assay
-
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Cancer cell autophagy, reprogrammed macrophages, and remodeled vasculature in glioblastoma triggers tumor immunity.
In Cancer Cell on 10 October 2022 by Chryplewicz, A., Scotton, J., et al.
PubMed
Glioblastoma (GBM) is poorly responsive to therapy and invariably lethal. One conceivable strategy to circumvent this intractability is to co-target distinctive mechanistic components of the disease, aiming to concomitantly disrupt multiple capabilities required for tumor progression and therapeutic resistance. We assessed this concept by combining vascular endothelial growth factor (VEGF) pathway inhibitors that remodel the tumor vasculature with the tricyclic antidepressant imipramine, which enhances autophagy in GBM cancer cells and unexpectedly reprograms immunosuppressive tumor-associated macrophages via inhibition of histamine receptor signaling to become immunostimulatory. While neither drug is efficacious as monotherapy, the combination of imipramine with VEGF pathway inhibitors orchestrates the infiltration and activation of CD8 and CD4 T cells, producing significant therapeutic benefit in several GBM mouse models. Inclusion up front of immune-checkpoint blockade with anti-programmed death-ligand 1 (PD-L1) in eventually relapsing tumors markedly extends survival benefit. The results illustrate the potential of mechanism-guided therapeutic co-targeting of disparate biological vulnerabilities in the tumor microenvironment.
-
-
-
Immunology and Microbiology
Blocking immune cell infiltration of the central nervous system to tame Neuroinflammation in Amyotrophic lateral sclerosis.
In Brain Behav Immun on 1 October 2022 by Garofalo, S., Cocozza, G., et al.
PubMed
Neuroinflammation is one of the main hallmarks of amyotrophic lateral sclerosis (ALS). Recently, peripheral immune cells were discovered as pivotal players that promptly participate in this process, speeding up neurodegeneration during progression of the disease. In particular, infiltrating T cells and natural killer cells release inflammatory cytokines that switch glial cells toward a pro-inflammatory/detrimental phenotype, and directly attack motor neurons with specific ligand-receptor signals. Here, we assessed the presence of lymphocytes in the spinal cord of sporadic ALS patients. Furthermore, we demonstrate that blocking the extravasation of immune cells in the central nervous system using Natalizumab (NAT), an antibody for the α4 integrin, reduces the level of interferon-γ in the spinal cord of ALS mouse models, such as the hSOD1G93A and TDP43A315T mice, modifying microglia and astrocytes phenotype, increasing motor neuron number and prolonging the survival time. Taken together, our results establish a central role for the immune cells as drivers of inflammation in ALS.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
Monocytes transition to macrophages within the inflamed vasculature via monocyte CCR2 and endothelial TNFR2.
In J Exp Med on 2 May 2022 by Mysore, V., Tahir, S., et al.
PubMed
Monocytes undergo phenotypic and functional changes in response to inflammatory cues, but the molecular signals that drive different monocyte states remain largely undefined. We show that monocytes acquire macrophage markers upon glomerulonephritis and may be derived from CCR2+CX3CR1+ double-positive monocytes, which are preferentially recruited, dwell within glomerular capillaries, and acquire proinflammatory characteristics in the nephritic kidney. Mechanistically, the transition to immature macrophages begins within the vasculature and relies on CCR2 in circulating cells and TNFR2 in parenchymal cells, findings that are recapitulated in vitro with monocytes cocultured with TNF-TNFR2-activated endothelial cells generating CCR2 ligands. Single-cell RNA sequencing of cocultures defines a CCR2-dependent monocyte differentiation path associated with the acquisition of immune effector functions and generation of CCR2 ligands. Immature macrophages are detected in the urine of lupus nephritis patients, and their frequency correlates with clinical disease. In conclusion, CCR2-dependent functional specialization of monocytes into macrophages begins within the TNF-TNFR2-activated vasculature and may establish a CCR2-based autocrine, feed-forward loop that amplifies renal inflammation.
-
-
Endothelial PERK-ATF4-JAG1 axis activated by T-ALL remodels bone marrow vascular niche.
In Theranostics on 12 April 2022 by Liu, C., Chen, Q., et al.
PubMed
The endoplasmic reticulum unfolded protein response (UPR) is a conserved adaptive signaling in ER homeostasis and has emerged as critical in highly proliferating cells and potential treatment target for acute T-cell lymphoblastic leukemia (T-ALL). Methods: in this study, we assessed the transcriptomic and phenotypic alterations in UPR response of the bone marrow endothelial cells (ECs) in mice engrafted with T-ALL and in bone marrow specimens from patients who have T-ALL. We used PERK inhibitor and generated endothelial specific PERK knockout mice to study the impact of PERK on leukemia progression and hematopoiesis. We performed chromatin immunoprecipitation (ChIP) to study the mechanistic regulation of JAG1 by ATF4. We characterized small extracellular vesicles (SEV) from leukemia-developing mice and studied the effect of SEVs on EC function. Results: we found that T-ALL development induced a robust activation of protein kinase RNA-like endoplasmic reticulum kinase (PERK)-dominant UPR in the bone marrow endothelial vascular niche. The activation of PERK-eIF2a-ATF4 axis remodels the vascular niche, upregulates angiogenic factors including VEGFα and ATF4-regulated JAG1, and suppresses the expression of SCF and CXCL12, which are important to HSC maintenance and regeneration. Further, targeting endothelial PERK significantly improved T-ALL outcome. EC-specific deletion of PERK abolished the aberrant JAG1 up-regulation, improved HSC maintenance, promoted leukemia apoptosis, and improved overall survival. Finally, we showed that small extracellular vesicles are critical mediators of endothelial PERK-eIF2a-ATF4 activation and JAG1 up-regulation in leukemia. Corroborating animal model studies, activation of PERK-ATF4-JAG1 is prominent in human T-ALL bone marrow and T-ALL xenografts. Conclusion: our studies thus revealed for the first time that the leukemia-initiated PERK-ATF4-JAG1 axis plays a critical role in the remodeling of the bone marrow vascular niche and that targeting vascular niche UPR is a potential therapeutic opportunity in T-ALL.
-
-
Immunodepletion
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Recruitment of α4β7 monocytes and neutrophils to the brain in experimental colitis is associated with elevated cytokines and anxiety-like behavior.
In J Neuroinflammation on 4 April 2022 by Cluny, N. L., Nyuyki, K. D., et al.
PubMed
Behavioral comorbidities, such as anxiety and depression, are a prominent feature of IBD. The signals from the inflamed gut that cause changes in the brain leading to these behavioral comorbidities remain to be fully elucidated. We tested the hypothesis that enhanced leukocyte-cerebral endothelial cell interactions occur in the brain in experimental colitis, mediated by α4β7 integrin, to initiate neuroimmune activation and anxiety-like behavior.
-