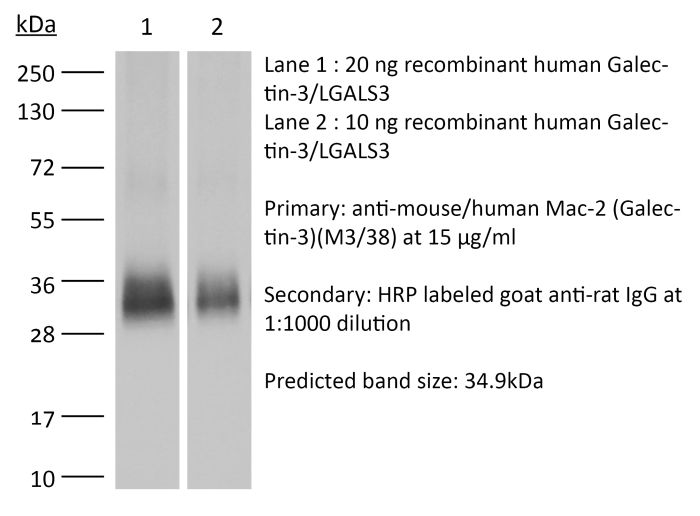
InVivoMAb anti-mouse/human Mac-2 (Galectin-3)
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | C57BL/6 mouse macrophage glycoproteins |
| Reported Applications |
Western blot Immunofluorescence Immunohistochemistry (paraffin) Immunoprecipitation |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Immunohistochemistry (paraffin)
Pedersen K, Nielsen MA, Juul-Madsen K, Hvid M, Deleuran B, Greisen SR (2023). "Galectin-3 interacts with PD-1 and counteracts the PD-1 pathway-driven regulation of T cell and osteoclast activity in Rheumatoid Arthritis" Scand J Immunol 97(2):e13245.
PubMed
Rheumatoid arthritis (RA) is an autoimmune disease characterized by joint inflammation and bone erosions. The glycosylated programmed death-1 (PD-1) receptor plays an important role in regulating immune responses and maintaining tolerance. In this study, we focus on two features observed in RA: impaired PD-1 signalling and Galectin-3 (Gal-3) upregulation. We hypothesize that Gal-3 binds PD-1 and PD-1 ligands, potentially contributing to impaired PD-1 signalling. PD-1 and Gal-3 levels in RA synovial fluid (SF) and plasma were evaluated by ELISA. PD-1 and Gal-3 interaction was examined by Surface Plasmon Resonance and ELISA. PD-1, PD-L1 and Gal-3 expression on mononuclear cells from SF and peripheral blood as well as fibroblast-like synoviocytes were examined by flow cytometry. Effects of Gal-3 and PD-L1 on osteoclast formation was evaluated by tartrate-resistant acid phosphatase assay. We show that Gal-3 binds PD-1 and PD-L1. Results demonstrated high expression of PD-1 and Gal-3 on mononuclear cells, especially from SF. Gal-3 inhibited PD-1 signalling when PD-L1 was present. Furthermore, a role of Gal-3 in osteoclast formation was observed in vitro, both directly but also through PD-1:PD-L1 inhibition. Effects of Gal-3 on the PD-1 signalling axis are proposed to be inhibitory, meaning high Gal-3 levels in the complex synovial microenvironment are not desirable in RA. Preventing Gal-3's inhibitory role on PD-1 signalling could, therefore, be a therapeutic target in RA by affecting inflammatory T cell responses and osteoclasts.
Immunofluorescence
Melo FH, Butera D, Junqueira Mde S, Hsu DK, da Silva AM, Liu FT, Santos MF, Chammas R (2011). "The promigratory activity of the matricellular protein galectin-3 depends on the activation of PI-3 kinase" PLoS One 6(12):e29313.
PubMed
Expression of galectin-3 is associated with sarcoma progression, invasion and metastasis. Here we determined the role of extracellular galectin-3 on migration of sarcoma cells on laminin-111. Cell lines from methylcholanthrene-induced sarcomas from both wild type and galectin-3(-/-) mice were established. Despite the presence of similar levels of laminin-binding integrins on the cell surface, galectin-3(-/-) sarcoma cells were more adherent and less migratory than galectin-3(+/+) sarcoma cells on laminin-111. When galectin-3 was transiently expressed in galectin-3(-/-) sarcoma cells, it inhibited cell adhesion and stimulated the migratory response to laminin in a carbohydrate-dependent manner. Extracellular galectin-3 led to the recruitment of SHP-2 phosphatase to focal adhesion plaques, followed by a decrease in the amount of phosphorylated FAK and phospho-paxillin in the lamellipodia of migrating cells. The promigratory activity of extracellular galectin-3 was inhibitable by wortmannin, implicating the activation of a PI-3 kinase dependent pathway in the galectin-3 triggered disruption of adhesion plaques, leading to sarcoma cell migration on laminin-111.
Western Blot
Immunoprecipitation
Rosenberg I, Cherayil BJ, Isselbacher KJ, Pillai S (1991). "Mac-2-binding glycoproteins. Putative ligands for a cytosolic beta-galactoside lectin" J Biol Chem 266(28):18731-6.
PubMed
Mac-2, a galactose-binding lectin secretion by activated macrophages, is the major non-integrin laminin-binding protein in these cells. Mac-2 is also expressed by epithelial cells in the intestine and kidney. We wished to identify intestinal glycoproteins other than laminin that have a high affinity for Mac-2 and that could be considered as candidate ligands or partners for this lectin in intestinal epithelium. Certain lines of human colon adenocarcinoma cells produce two Mac-2-binding glycoproteins (M2BP-1 and M2BP-2) that were identified by their avid association with Mac-2 following detergent lysis and immunoprecipitation. These glycoproteins do not share a common epitope with Mac-2, and the interaction between Mac-2 and these proteins is mediated through the carbohydrate-binding domain of Mac-2 and sugar moieties on M2BP-1 and M2BP-2. M2BP-1 (98 kDa) and M2BP-2 (70 kDa) were purified by immunoaffinity chromatography and were specifically eluted with either galactose or lactose. Peptide maps revealed that M2BP-1 and M2BP-2 are structurally related. M2BP-1 is secreted and could conceivably associate with Mac-2 extracellularly. N-terminal sequence analysis of M2BP-2 suggests that these glycoproteins represent a unique subset of candidate ligands for this mammalian beta-galactoside lectin.