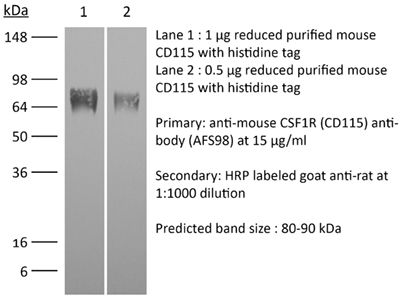
InVivoMAb anti-mouse CSF1R (CD115)
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Not available or unknown |
| Reported Applications |
in vivo macrophage depletion in vitro CSF1R neutralization in vivo monocyte depletion Flow cytometry Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687699 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo macrophage depletion
Bauche, D., et al (2018). "LAG3(+) Regulatory T Cells Restrain Interleukin-23-Producing CX3CR1(+) Gut-Resident Macrophages during Group 3 Innate Lymphoid Cell-Driven Colitis" Immunity 49(2): 342-352 e345.
PubMed
Interleukin-22 (IL-22)-producing group 3 innate lymphoid cells (ILC3) maintains gut homeostasis but can also promote inflammatory bowel disease (IBD). The regulation of ILC3-dependent colitis remains to be elucidated. Here we show that Foxp3(+) regulatory T cells (Treg cells) prevented ILC3-mediated colitis in an IL-10-independent manner. Treg cells inhibited IL-23 and IL-1beta production from intestinal-resident CX3CR1(+) macrophages but not CD103(+) dendritic cells. Moreover, Treg cells restrained ILC3 production of IL-22 through suppression of CX3CR1(+) macrophage production of IL-23 and IL-1beta. This suppression was contact dependent and was mediated by latent activation gene-3 (LAG-3)-an immune checkpoint receptor-expressed on Treg cells. Engagement of LAG-3 on MHC class II drove profound immunosuppression of CX3CR1(+) tissue-resident macrophages. Our study reveals that the health of the intestinal mucosa is maintained by an axis driven by Treg cells communication with resident macrophages that withhold inflammatory stimuli required for ILC3 function.
in vivo macrophage depletion
Gordon, S. R., et al (2017). "PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity" Nature 545(7655): 495-499.
PubMed
Programmed cell death protein 1 (PD-1) is an immune checkpoint receptor that is upregulated on activated T cells for the induction of immune tolerance. Tumour cells frequently overexpress the ligand for PD-1, programmed cell death ligand 1 (PD-L1), facilitating their escape from the immune system. Monoclonal antibodies that block the interaction between PD-1 and PD-L1, by binding to either the ligand or receptor, have shown notable clinical efficacy in patients with a variety of cancers, including melanoma, colorectal cancer, non-small-cell lung cancer and Hodgkin’s lymphoma. Although it is well established that PD-1-PD-L1 blockade activates T cells, little is known about the role that this pathway may have in tumour-associated macrophages (TAMs). Here we show that both mouse and human TAMs express PD-1. TAM PD-1 expression increases over time in mouse models of cancer and with increasing disease stage in primary human cancers. TAM PD-1 expression correlates negatively with phagocytic potency against tumour cells, and blockade of PD-1-PD-L1 in vivo increases macrophage phagocytosis, reduces tumour growth and lengthens the survival of mice in mouse models of cancer in a macrophage-dependent fashion. This suggests that PD-1-PD-L1 therapies may also function through a direct effect on macrophages, with substantial implications for the treatment of cancer with these agents.
in vivo macrophage depletion
Moynihan, K. D., et al (2016). "Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses" Nat Med. doi : 10.1038/nm.4200.
PubMed
Checkpoint blockade with antibodies specific for cytotoxic T lymphocyte-associated protein (CTLA)-4 or programmed cell death 1 (PDCD1; also known as PD-1) elicits durable tumor regression in metastatic cancer, but these dramatic responses are confined to a minority of patients. This suboptimal outcome is probably due in part to the complex network of immunosuppressive pathways present in advanced tumors, which are unlikely to be overcome by intervention at a single signaling checkpoint. Here we describe a combination immunotherapy that recruits a variety of innate and adaptive immune cells to eliminate large tumor burdens in syngeneic tumor models and a genetically engineered mouse model of melanoma; to our knowledge tumors of this size have not previously been curable by treatments relying on endogenous immunity. Maximal antitumor efficacy required four components: a tumor-antigen-targeting antibody, a recombinant interleukin-2 with an extended half-life, anti-PD-1 and a powerful T cell vaccine. Depletion experiments revealed that CD8+ T cells, cross-presenting dendritic cells and several other innate immune cell subsets were required for tumor regression. Effective treatment induced infiltration of immune cells and production of inflammatory cytokines in the tumor, enhanced antibody-mediated tumor antigen uptake and promoted antigen spreading. These results demonstrate the capacity of an elicited endogenous immune response to destroy large, established tumors and elucidate essential characteristics of combination immunotherapies that are capable of curing a majority of tumors in experimental settings typically viewed as intractable.
in vivo macrophage depletion
Arnold, I. C., et al (2015). "CD11c monocyte/macrophages promote chronic Helicobacter hepaticus-induced intestinal inflammation through the production of IL-23" Mucosal Immunol. doi : 10.1038/mi.2015.65.
PubMed
In inflammatory bowel diseases, a breakdown in host microbial interactions accompanies sustained activation of immune cells in the gut. Functional studies suggest a key role for interleukin-23 (IL-23) in orchestrating intestinal inflammation. IL-23 can be produced by various mononuclear phagocytes (MNPs) following acute microbial stimulation, but little is known about the key cellular sources of IL-23 that drive chronic intestinal inflammation. Here we have addressed this question using a physiological model of bacteria-driven colitis. By combining conditional gene ablation and gene expression profiling, we found that IL-23 production by CD11c+ MNPs was essential to trigger intestinal immunopathology and identified MHCII+ monocytes and macrophages as the major source of IL-23. Expression of IL-23 by monocytes was acquired during their differentiation in the intestine and correlated with the expression of major histocompatibility complex class II (MHCII) and CD64. In contrast, Batf3-dependent CD103+ CD11b- dendritic cells were dispensable for bacteria-induced colitis in this model. These studies reinforce the pathogenic role of monocytes in dysregulated responses to intestinal bacteria and identify production of IL-23 as a key component of this response. Further understanding of the functional sources of IL-23 in diverse forms of intestinal inflammation may lead to novel therapeutic strategies aimed at interrupting IL-23-driven immune pathology.Mucosal Immunology advance online publication 5 August 2015. doi:10.1038/mi.2015.65.
in vivo macrophage depletion
Kaminsky, L. W., et al (2015). "Redundant Function of Plasmacytoid and Conventional Dendritic Cells Is Required To Survive a Natural Virus Infection" J Virol 89(19): 9974-9985.
PubMed
Viruses that spread systemically from a peripheral site of infection cause morbidity and mortality in the human population. Innate myeloid cells, including monocytes, macrophages, monocyte-derived dendritic cells (mo-DC), and dendritic cells (DC), respond early during viral infection to control viral replication, reducing virus spread from the peripheral site. Ectromelia virus (ECTV), an orthopoxvirus that naturally infects the mouse, spreads systemically from the peripheral site of infection and results in death of susceptible mice. While phagocytic cells have a requisite role in the response to ECTV, the requirement for individual myeloid cell populations during acute immune responses to peripheral viral infection is unclear. In this study, a variety of myeloid-specific depletion methods were used to dissect the roles of individual myeloid cell subsets in the survival of ECTV infection. We showed that DC are the primary producers of type I interferons (T1-IFN), requisite cytokines for survival, following ECTV infection. DC, but not macrophages, monocytes, or granulocytes, were required for control of the virus and survival of mice following ECTV infection. Depletion of either plasmacytoid DC (pDC) alone or the lymphoid-resident DC subset (CD8alpha(+) DC) alone did not confer lethal susceptibility to ECTV. However, the function of at least one of the pDC or CD8alpha(+) DC subsets is required for survival of ECTV infection, as mice depleted of both populations were susceptible to ECTV challenge. The presence of at least one of these DC subsets is sufficient for cytokine production that reduces ECTV replication and virus spread, facilitating survival following infection. IMPORTANCE: Prior to the eradication of variola virus, the orthopoxvirus that causes smallpox, one-third of infected people succumbed to the disease. Following successful eradication of smallpox, vaccination rates with the smallpox vaccine have significantly dropped. There is now an increasing incidence of zoonotic orthopoxvirus infections for which there are no effective treatments. Moreover, the safety of the smallpox vaccine is of great concern, as complications may arise, resulting in morbidity. Like many viruses that cause significant human diseases, orthopoxviruses spread from a peripheral site of infection to become systemic. This study elucidates the early requirement for innate immune cells in controlling a peripheral infection with ECTV, the causative agent of mousepox. We report that there is redundancy in the function of two innate immune cell subsets in controlling virus spread early during infection. The viral control mediated by these cell subsets presents a potential target for therapies and rational vaccine design.
in vivo monocyte depletion
Naik, S., et al (2015). "Commensal-dendritic-cell interaction specifies a unique protective skin immune signature" Nature 520(7545): 104-108.
PubMed
The skin represents the primary interface between the host and the environment. This organ is also home to trillions of microorganisms that play an important role in tissue homeostasis and local immunity. Skin microbial communities are highly diverse and can be remodelled over time or in response to environmental challenges. How, in the context of this complexity, individual commensal microorganisms may differentially modulate skin immunity and the consequences of these responses for tissue physiology remains unclear. Here we show that defined commensals dominantly affect skin immunity and identify the cellular mediators involved in this specification. In particular, colonization with Staphylococcus epidermidis induces IL-17A(+) CD8(+) T cells that home to the epidermis, enhance innate barrier immunity and limit pathogen invasion. Commensal-specific T-cell responses result from the coordinated action of skin-resident dendritic cell subsets and are not associated with inflammation, revealing that tissue-resident cells are poised to sense and respond to alterations in microbial communities. This interaction may represent an evolutionary means by which the skin immune system uses fluctuating commensal signals to calibrate barrier immunity and provide heterologous protection against invasive pathogens. These findings reveal that the skin immune landscape is a highly dynamic environment that can be rapidly and specifically remodelled by encounters with defined commensals, findings that have profound implications for our understanding of tissue-specific immunity and pathologies.
in vitro CSF-R1 neutralization
Sheng, K. C., et al (2014). "IL-3 and CSF-1 interact to promote generation of CD11c+ IL-10-producing macrophages" PLoS One 9(4): e95208.
PubMed
Unraveling the mechanisms of hematopoiesis regulated by multiple cytokines remains a challenge in hematology. IL-3 is an allergic cytokine with the multilineage potential, while CSF-1 is produced in the steady state with restricted lineage coverage. Here, we uncovered an instructive role of CSF-1 in IL-3-mediated hematopoiesis. CSF-1 significantly promoted IL-3-driven CD11c+ cell expansion and dampened basophil and mast cell generation from C57BL/6 bone marrow. Further studies indicated that the CSF-1/CSF-1R axis contributed significantly to IL-3-induced CD11c+ cell generation through enhancing c-Fos-associated monopoiesis. CD11c+ cells induced by IL-3 or IL-3/CSF-1 were competent in cellular maturation and endocytosis. Both IL-3 and IL-3/CSF-1 cells lacked classical dendritic cell appearance and resembled macrophages in morphology. Both populations produced a high level of IL-10, in addition to IL-1, IL-6 and TNFalpha, in response to LPS, and were relatively poor T cell stimulators. Collectively, these findings reveal a role for CSF-1 in mediating the IL-3 hematopoietic pathway through monopoiesis, which regulates expansion of CD11c+ macrophages.
in vivo monocyte depletion
Greter, M., et al (2012). "GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells" Immunity 36(6): 1031-1046.
PubMed
GM-CSF (Csf-2) is a critical cytokine for the in vitro generation of dendritic cells (DCs) and is thought to control the development of inflammatory DCs and resident CD103(+) DCs in some tissues. Here we showed that in contrast to the current understanding, Csf-2 receptor acts in the steady state to promote the survival and homeostasis of nonlymphoid tissue-resident CD103(+) and CD11b(+) DCs. Absence of Csf-2 receptor on lung DCs abrogated the induction of CD8(+) T cell immunity after immunization with particulate antigens. In contrast, Csf-2 receptor was dispensable for the differentiation and innate function of inflammatory DCs during acute injuries. Instead, inflammatory DCs required Csf-1 receptor for their development. Thus, Csf-2 is important in vaccine-induced CD8(+) T cell immunity through the regulation of nonlymphoid tissue DC homeostasis rather than control of inflammatory DCs in vivo.
Flow Cytometry
Li, W., et al (2012). "Intravital 2-photon imaging of leukocyte trafficking in beating heart" J Clin Invest 122(7): 2499-2508.
PubMed
Two-photon intravital microscopy has substantially broadened our understanding of tissue- and organ-specific differences in the regulation of inflammatory responses. However, little is known about the dynamic regulation of leukocyte recruitment into inflamed heart tissue, largely due to technical difficulties inherent in imaging moving tissue. Here, we report a method for imaging beating murine hearts using intravital 2-photon microscopy. Using this method, we visualized neutrophil trafficking at baseline and during inflammation. Ischemia reperfusion injury induced by transplantation or transient coronary artery ligation led to recruitment of neutrophils to the heart, their extravasation from coronary veins, and infiltration of the myocardium where they formed large clusters. Grafting hearts containing mutant ICAM-1, a ligand important for neutrophil recruitment, reduced the crawling velocities of neutrophils within vessels, and markedly inhibited their extravasation. Similar impairment was seen with the inhibition of Mac-1, a receptor for ICAM-1. Blockade of LFA-1, another ICAM-1 receptor, prevented neutrophil adherence to endothelium and extravasation in heart grafts. As inflammatory responses in the heart are of great relevance to public health, this imaging approach holds promise for studying cardiac-specific mechanisms of leukocyte recruitment and identifying novel therapeutic targets for treating heart disease.
Flow Cytometry
Tagliani, E., et al (2011). "Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1" J Exp Med 208(9): 1901-1916.
PubMed
Tissue macrophages (Mphis) and dendritic cells (DCs) play essential roles in tissue homeostasis and immunity. How these cells are maintained at their characteristic densities in different tissues has remained unclear. Aided by a novel flow cytometric technique for assessing relative rates of blood-borne precursor recruitment, we examined Mphi and DC population dynamics in the pregnant mouse uterus, where rapid tissue growth facilitated a dissection of underlying regulatory mechanisms. We demonstrate how Mphi dynamics, and thus Mphi tissue densities, are locally controlled by CSF-1, a pleiotropic growth factor whose in situ level of activity varied widely between uterine tissue layers. CSF-1 acted in part by inducing Mphi proliferation and in part by stimulating the extravasation of Ly6C(hi) monocytes (Mos) that served as Mphi precursors. Mo recruitment was dependent on the production of CCR2 chemokine receptor ligands by uterine Mphis in response to CSF-1. Unexpectedly, a parallel CSF-1-regulated, but CCR2-independent pathway influenced uterine DC tissue densities by controlling local pre-DC extravasation rates. Together, these data provide cellular and molecular insight into the regulation of Mphi tissue densities under noninflammatory conditions and reveal a central role for CSF-1 in the coordination of Mphi and DC homeostasis.
Lim, A. K., et al (2009). "Antibody blockade of c-fms suppresses the progression of inflammation and injury in early diabetic nephropathy in obese db/db mice" Diabetologia 52(8): 1669-1679.
PubMed
AIMS/HYPOTHESIS: Macrophage-mediated renal injury plays an important role in the development of diabetic nephropathy. Colony-stimulating factor (CSF)-1 is a cytokine that is produced in diabetic kidneys and promotes macrophage accumulation, activation and survival. CSF-1 acts exclusively through the c-fms receptor, which is only expressed on cells of the monocyte-macrophage lineage. Therefore, we used c-fms blockade as a strategy to selectively target macrophage-mediated injury during the progression of diabetic nephropathy. METHODS: Obese, type 2 diabetic db/db BL/KS mice with established albuminuria were treated with a neutralising anti-c-fms monoclonal antibody (AFS98) or isotype matched control IgG from 12 to 18 weeks of age and examined for renal injury. RESULTS: Treatment with AFS98 did not affect obesity, hyperglycaemia, circulating monocyte levels or established albuminuria in db/db mice. However, AFS98 did prevent glomerular hyperfiltration and suppressed variables of inflammation in the diabetic kidney, including kidney macrophages (accumulation, activation and proliferation), chemokine CC motif ligand 2 levels (mRNA and urine protein), kidney activation of proinflammatory pathways (c-Jun amino-terminal kinase and activating transcription factor 2) and Tnf-alpha (also known as Tnf) mRNA levels. In addition, AFS98 decreased the tissue damage caused by macrophages including tubular injury (apoptosis and hypertrophy), interstitial damage (cell proliferation and myofibroblast accrual) and renal fibrosis (Tgf-beta1 [also known as Tgfb1] and Col4a1 mRNA). CONCLUSIONS/INTERPRETATION: Blockade of c-fms can suppress the progression of established diabetic nephropathy in db/db mice by targeting macrophage-mediated injury.
Product Citations
-
CircRNA circ_0004058 Modulates Early Brain Injury in Subarachnoid Hemorrhage Through miR-221-3p and VE1 Activation Pathway.
In Transl Stroke Res on 1 December 2025 by Gu, H., Cai, Y., et al.
PubMed
Subarachnoid hemorrhage (SAH) frequently results in early brain injury (EBI), which remains a major barrier to favorable neurological recovery. Understanding the molecular underpinnings of EBI is crucial for developing targeted therapeutics. Circular RNAs (circRNAs) have emerged as influential molecular players in various brain injury contexts. This study focuses on one such molecule, circ_0004058, examining its impact on EBI through interaction with miR-221-3p and the VE1 signaling pathway. Utilizing an established SAH rodent model, our team conducted a detailed investigation of the expression patterns and interactions involving circ_0004058. Our analyses revealed a significant post-SAH upregulation of circ_0004058, which affected miR-221-3p activity and VE1 signaling. Furthermore, functional modulation of circ_0004058 expression altered the severity of EBI, presenting evidence that it serves as a critical determinant in the injury process. The results suggest that circ_0004058 holds promise as a therapeutic target, offering new possibilities for the development of strategies to mitigate SAH-induced brain damage. Through this study, circ_0004058 is highlighted not only as a biomarker but also as a possible avenue for therapeutic modulation in SAH management.
-
-
Immunology and Microbiology
-
Cardiovascular biology
Macrophage depletion lowers blood pressure and reduces renal fibrosis progression in existing hypertension mice model.
In J Physiol Sci on 1 November 2025 by Peter, J. K., Umene, R., et al.
PubMed
Uncontrolled hypertension is a global health issue with 40 % of hypertensive patients not achieving blood pressure control with current therapies. Previously, we demonstrated renal macrophage infiltration during hypertension development with macrophage depletion leading to reduced blood pressure and renal fibrosis. However, the effect of macrophage depletion in existing hypertension has not been evaluated. We induced hypertension in mice then depleted macrophages and assessed blood pressure and renal fibrosis. Separately induced hypertension and assessed renal macrophage population and fibrosis early in hypertension. Results showed increased renal macrophage, Acta2 early in hypertension development. Macrophage depletion led to reduced blood pressure in the hypertensive mice, decreased kidney Col1a1, Acta2, Col3a1 and Fn1. This study shows that renal macrophage infiltration and fibrosis begin early in hypertension development and depleting macrophages in hypertension reduces blood pressure and suppress renal fibrosis. This shows macrophages are a potential target in treatment of hypertension.
-
-
-
Immunology and Microbiology
-
Cancer Research
Synergically enhanced anti-tumor immunity of in vivo panCAR by circRNA vaccine boosting.
In Cell Rep Med on 19 August 2025 by Wang, Y., Lin, L., et al.
PubMed
Chimeric antigen receptor (CAR) T cell therapy has shown promise in treating hematologic malignancies, but it still faces challenges, including high costs, a time-consuming manufacturing process, and the necessity of lymphodepletion. Here, we generate circular RNAs (circRNAs) encoding CAR proteins, referred to as circRNACAR, which mediates remarkable tumor killing in human primary T cells. We demonstrate that circRNACAR, delivered with immunocyte-tropic lipid nanoparticles (LNPs), can form in vivo panCAR cells (CAR-T, CAR-natural killer [NK], and CAR-macrophage), significantly inhibit tumor growth, and reshape the tumor microenvironment in mice. Importantly, combining in vivo panCAR with circRNA-based vaccines encoding the corresponding HER2 antigens exhibits synergistically enhanced anti-tumor immunity. Notably, circRNACAR can in return boost the level of vaccination-elicited HER2-specific antibodies, mediating effective killing of tumor cells by macrophages. In combination with vaccination, in vivo panCAR demonstrates a synergistic enhancement of anti-tumor immunity across various mouse models, thereby establishing a framework for the synergistic in vivo panCAR-VAC immunotherapy.
-
-
-
Immunology and Microbiology
-
Cancer Research
Orchestrating intratumoral DC-T cell immunity for enhanced tumor control via radiotherapy-activated TLR7/8 prodrugs in mice.
In Nat Commun on 1 July 2025 by Yin, X., Ding, Z., et al.
PubMed
Optimizing intratumoral dendritic cell (DC)-T cell responses is pivotal for effective cancer immunotherapy. However, the mechanistic governing these dynamics within the tumor microenvironment (TME) remains unclear, and strategies to improve their therapeutic potential are underexplored. Here, we show that precise radiotherapy activates the pro-TLR7/8 agonist imidazoquinoline (IMDQ) locally in preclinical tumor models, stimulating DCs to elicit T cell immunity without the need for further recruitment or causing systemic toxicity. Mechanistically, this synergistic approach triggers type I interferon via STING and MyD88 signaling pathways, strengthening local immune responses. Importantly, we reveal that fractionated, low-dose radiotherapy can effectively optimize local DC-T cell dynamics to control the irradiated tumor, while also promoting abscopal effect. Thus, our findings underscore the critical role of harnessing intratumoral DCs to reinvigorate pre-existing T cell immunity and provide mechanistic insights into improving both local and distal tumor control, opening new avenues for advancing cancer immunotherapy.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Genetics
Histone methyltransferase ASH1L primes metastases and metabolic reprogramming of macrophages in the bone niche.
In Nat Commun on 20 May 2025 by Meng, C., Lin, K., et al.
PubMed
Bone metastasis is a major cause of cancer death; however, the epigenetic determinants driving this process remain elusive. Here, we report that histone methyltransferase ASH1L is genetically amplified and is required for bone metastasis in men with prostate cancer. ASH1L rewires histone methylations and cooperates with HIF-1α to induce pro-metastatic transcriptome in invading cancer cells, resulting in monocyte differentiation into lipid-associated macrophage (LA-TAM) and enhancing their pro-tumoral phenotype in the metastatic bone niche. We identified IGF-2 as a direct target of ASH1L/HIF-1α and mediates LA-TAMs' differentiation and phenotypic changes by reprogramming oxidative phosphorylation. Pharmacologic inhibition of the ASH1L-HIF-1α-macrophages axis elicits robust anti-metastasis responses in preclinical models. Our study demonstrates epigenetic alterations in cancer cells reprogram metabolism and features of myeloid components, facilitating metastatic outgrowth. It establishes ASH1L as an epigenetic driver priming metastasis and macrophage plasticity in the bone niche, providing a bona fide therapeutic target in metastatic malignancies.
-
-
-
Cancer Research
Ultra-high dose rate radiotherapy overcomes radioresistance in head and neck squamous cell carcinoma.
In Signal Transduct Target Ther on 3 March 2025 by Li, H. S., Tang, R., et al.
PubMed
Radiotherapy (RT) resistance in head and neck squamous cell carcinoma (HNSCC) significantly hampers local control and patient prognosis. This study investigated the efficacy and molecular mechanisms of high-energy X-ray-based ultra-high dose rate radiotherapy (UHDR-RT) in overcoming RT resistance. The established RT-resistant HNSCC cell lines and animal models were subjected to UHDR-RT or conventional RT (Conv-RT) via a high-power rhodotron accelerator. Cellular assays assessed the malignant phenotype, viability, and degree of DNA damage, whereas in vivo evaluations focused on tumor proliferation and the tumor immune microenvironment (TiME). Transcriptome sequencing and Olink proteomics were employed to explore the underlying mechanisms involved. In vitro experiments indicated that UHDR-RT suppressed radioresistant cell proliferation and invasion, while promoting apoptosis and exacerbating DNA damage. In contrast, its efficacy in radiosensitive cells was comparable to that of Conv-RT. In vivo studies using patient-derived xenograft nude mice models demonstrated that UHDR-RT only partially reversed RT resistance. Transcriptomic and proteomic analyses of C57BL/6J mice models revealed the predominant role of TiME modulating in reversing radioresistance. Immunofluorescence and flow cytometry confirmed increased CD8+ T cells and an increased M1/M2 macrophage ratio post-UHDR-RT. Mechanistically, UHDR-RT activated CD8+ T cells, which stimulated M1 macrophages through paracrine IFN-γ signaling, thereby enhancing TiME activation. Furthermore, the activated M1 macrophages secreted CXCL9, which in turn reactivated CD8+ T cells, forming a feedforward loop that amplified TiME activation. This study elucidates the dual role of UHDR-RT in directly inducing DNA damage and modulating the TiME, highlighting its potential in treating radioresistant HNSCC.
-
-
-
Immunology and Microbiology
-
Cancer Research
Single-cell data-driven design of armed oncolytic virus to boost cooperative innate-adaptive immunity against cancer.
In Mol Ther on 5 February 2025 by Zhao, J., Wang, H., et al.
PubMed
Oncolytic viruses have been considered promising cancer immunotherapies. However, oncovirotherapy agents impart durable responses in only a subset of cancer patients. Thus, exploring the cellular and molecular mechanisms underlying the heterogeneous responses in patients can provide guidance to develop more effective oncolytic virus therapies. Single-cell RNA sequencing (scRNA-seq) analysis of tumors responsive and non-responsive to oncovirotherapy revealed signatures of the tumor immune microenvironment associated with immune response. Thus, we designed and constructed an armed oncolytic virus, OV-5A, that expressed five genes with non-redundant functions. OV-5A treatment exhibits robust immune response against various tumors in multiple mouse models, peripheral blood mononuclear cell -patient-derived xenograft models, organoid-immune cell co-culture systems, and patient tissue sections by activating a cooperative innate-adaptive immune response against tumor cells. scRNA-seq analysis of complete responders and partial responders to OV-5A treatment guided the design of combination therapy of OV-5A. This data-driven approach paves an innovative way to rationalize the design of oncolytic virus and multi-agent combination therapies.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
CD226+adipose tissue macrophages arise from MDP-derived monocytes and regulate lipid metabolism
In bioRxiv on 5 December 2024 by Gallerand, A., Caillot, Z., et al.
-
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Endocrinology and Physiology
Endothelial metabolic control of insulin sensitivity through resident macrophages.
In Cell Metab on 5 November 2024 by Zhang, J., Sjøberg, K. A., et al.
PubMed
Endothelial cells (ECs) not only form passive blood conduits but actively contribute to nutrient transport and organ homeostasis. The role of ECs in glucose homeostasis is, however, poorly understood. Here, we show that, in skeletal muscle, endothelial glucose transporter 1 (Glut1/Slc2a1) controls glucose uptake via vascular metabolic control of muscle-resident macrophages without affecting transendothelial glucose transport. Lowering endothelial Glut1 via genetic depletion (Glut1ΔEC) or upon a short-term high-fat diet increased angiocrine osteopontin (OPN/Spp1) secretion. This promoted resident muscle macrophage activation and proliferation, which impaired muscle insulin sensitivity. Consequently, co-deleting Spp1 from ECs prevented macrophage accumulation and improved insulin sensitivity in Glut1ΔEC mice. Mechanistically, Glut1-dependent endothelial glucose metabolic rewiring increased OPN in a serine metabolism-dependent fashion. Our data illustrate how the glycolytic endothelium creates a microenvironment that controls resident muscle macrophage phenotype and function and directly links resident muscle macrophages to the maintenance of muscle glucose homeostasis.
-
-
-
Immunology and Microbiology
-
Stem Cells and Developmental Biology
Dermal TRPV1 innervations engage a macrophage- and fibroblast-containing pathway to activate hair growth in mice.
In Dev Cell on 4 November 2024 by Ben-Shaanan, T. L., Knöpper, K., et al.
PubMed
Pain, detected by nociceptors, is an integral part of injury, yet whether and how it can impact tissue physiology and recovery remain understudied. Here, we applied chemogenetics in mice to locally activate dermal TRPV1 innervations in naive skin and found that it triggered new regenerative cycling by dormant hair follicles (HFs). This was preceded by rapid apoptosis of dermal macrophages, mediated by the neuropeptide calcitonin gene-related peptide (CGRP). TRPV1 activation also triggered a macrophage-dependent induction of osteopontin (Spp1)-expressing dermal fibroblasts. The neuropeptide CGRP and the extracellular matrix protein Spp1 were required for the nociceptor-triggered hair growth. Finally, we showed that epidermal abrasion injury induced Spp1-expressing dermal fibroblasts and hair growth via a TRPV1 neuron and CGRP-dependent mechanism. Collectively, these data demonstrated a role for TRPV1 nociceptors in orchestrating a macrophage and fibroblast-supported mechanism to promote hair growth and enabling the efficient restoration of this mechano- and thermo-protective barrier after wounding.
-
-
-
Immunology and Microbiology
Thymosin α1 reverses oncolytic adenovirus-induced M2 polarization of macrophages to improve antitumor immunity and therapeutic efficacy.
In Cell Rep Med on 15 October 2024 by Liu, K., Kong, L., et al.
PubMed
Although oncolytic adenoviruses are widely studied for their direct oncolytic activity and immunomodulatory role in cancer immunotherapy, the immunosuppressive feedback loop induced by oncolytic adenoviruses remains to be studied. Here, we demonstrate that type V adenovirus (ADV) induces the polarization of tumor-associated macrophages (TAMs) to the M2 phenotype and increases the infiltration of regulatory T cells (Tregs) in the tumor microenvironment (TME). By selectively compensating for these deficiencies, thymosin alpha 1 (Tα1) reprograms "M2-like" TAMs toward an antitumoral phenotype, thereby reprogramming the TME into a state more beneficial for antitumor immunity. Moreover, ADVTα1 is constructed by harnessing the merits of all the components for the aforementioned combinatorial therapy. Both exogenously supplied and adenovirus-produced Tα1 orchestrate TAM reprogramming and enhance the antitumor efficacy of ADV via CD8+ T cells, showing promising prospects for clinical translation. Our findings provide inspiration for improving oncolytic adenovirus combination therapy and designing oncolytic engineered adenoviruses.
-
-
Reduction of circulating IgE and allergens by a pH-sensitive antibody with enhanced FcγRIIb binding.
In Mol Ther on 2 October 2024 by Li, N., Gong, N., et al.
PubMed
Allergen-crosslinked IgE triggers allergy by interacting with its receptor on basophils and mast cells. The anti-IgE monoclonal antibody omalizumab can alleviate allergy by competing with the receptor for IgE binding. However, along with neutralization, omalizumab also inhibits IgE degradation, which is clinically associated with high-dose and total IgE accumulation problems. In this study, we have developed an IgE-eliminating antibody on the basis of omalizumab, which has pH-dependent Fabs and an Fc with high affinity for FcγRIIb. In mice, the antibody rapidly eliminated total serum IgE to baseline levels and caused lower free IgE levels than omalizumab. At low dosages, the antibody also exhibited favorable IgE elimination effects. In addition, the antibody can degrade the corresponding allergen with the removal of IgE, addressing the allergy from its source. Introduction of the M252Y/S254T/T256E (YTE) mutation into this antibody prolongs its serum half-life without reducing potency. Thus, this engineered antibody holds a promising therapeutic option for allergy patients. Mechanistic insights are also included in this study.
-
-
Neuroscience
FcγR- and CD9-dependent synapse engulfing microglia in the thalamus drives cognitive impairment following cortical brain injury
In bioRxiv on 23 September 2024 by Matoba, K., Kochi, T., et al.
-
-
-
Immunology and Microbiology
West Nile virus triggers intestinal dysmotility via T cell-mediated enteric nervous system injury.
In J Clin Invest on 29 August 2024 by Janova, H., Zhao, F. R., et al.
PubMed
Intestinal dysmotility syndromes have been epidemiologically associated with several antecedent bacterial and viral infections. To model this phenotype, we previously infected mice with the neurotropic flavivirus West Nile virus (WNV) and demonstrated intestinal transit defects. Here, we found that within 1 week of WNV infection, enteric neurons and glia became damaged, resulting in sustained reductions of neuronal cells and their networks of connecting fibers. Using cell-depleting antibodies, adoptive transfer experiments, and mice lacking specific immune cells or immune functions, we show that infiltrating WNV-specific CD4+ and CD8+ T cells damaged the enteric nervous system (ENS) and glia, which led to intestinal dysmotility; these T cells used multiple and redundant effector molecules including perforin and Fas ligand. In comparison, WNV-triggered ENS injury and intestinal dysmotility appeared to not require infiltrating monocytes, and damage may have been limited by resident muscularis macrophages. Overall, our experiments support a model in which antigen-specific T cell subsets and their effector molecules responding to WNV infection direct immune pathology against enteric neurons and supporting glia that results in intestinal dysmotility.
-
-
-
Immunology and Microbiology
Adipose tissue macrophage infiltration and hepatocyte stress increase GDF-15 throughout development of obesity to MASH.
In Nat Commun on 21 August 2024 by L'homme, L., Sermikli, B. P., et al.
PubMed
Plasma growth differentiation factor-15 (GDF-15) levels increase with obesity and metabolic dysfunction-associated steatotic liver disease (MASLD) but the underlying mechanism remains poorly defined. Using male mouse models of obesity and MASLD, and biopsies from carefully-characterized patients regarding obesity, type 2 diabetes (T2D) and MASLD status, we identify adipose tissue (AT) as the key source of GDF-15 at onset of obesity and T2D, followed by liver during the progression towards metabolic dysfunction-associated steatohepatitis (MASH). Obesity and T2D increase GDF15 expression in AT through the accumulation of macrophages, which are the main immune cells expressing GDF15. Inactivation of Gdf15 in macrophages reduces plasma GDF-15 concentrations and exacerbates obesity in mice. During MASH development, Gdf15 expression additionally increases in hepatocytes through stress-induced TFEB and DDIT3 signaling. Together, these results demonstrate a dual contribution of AT and liver to GDF-15 production in metabolic diseases and identify potential therapeutic targets to raise endogenous GDF-15 levels.
-
-
Comprehensive assembly of monoclonal and mixed antibody sequences
In bioRxiv on 10 August 2024 by Jiang, W., Xiong, Y., et al.
-
-
Cancer Research
-
Immunology and Microbiology
Reprogramming the tumor immune microenvironment using engineered dual-drug loaded Salmonella.
In Nat Commun on 6 August 2024 by Nguyen, D. H., You, S. H., et al.
PubMed
Synergistic combinations of immunotherapeutic agents can improve the performance of anti-cancer therapies but may lead to immune-mediated adverse effects. These side-effects can be overcome by using a tumor-specific delivery system. Here, we report a method of targeted immunotherapy using an attenuated Salmonella typhimurium (SAM-FC) engineered to release dual payloads: cytolysin A (ClyA), a cytolytic anti-cancer agent, and Vibrio vulnificus flagellin B (FlaB), a potent inducer of anti-tumor innate immunity. Localized secretion of ClyA from SAM-FC induces immunogenic cancer cell death and promotes release of tumor-specific antigens and damage-associated molecular patterns, which establish long-term antitumor memory. Localized secretion of FlaB promotes phenotypic and functional remodeling of intratumoral macrophages that markedly inhibits tumor metastasis in mice bearing tumors of mouse and human origin. Both primary and metastatic tumors from bacteria-treated female mice are characterized by massive infiltration of anti-tumorigenic innate immune cells and activated tumor-specific effector/memory T cells; however, the percentage of immunosuppressive cells is low. Here, we show that SAM-FC induces functional reprogramming of the tumor immune microenvironment by activating both the innate and adaptive arms of the immune system and can be used for targeted delivery of multiple immunotherapeutic payloads for the establishment of potent and long-lasting antitumor immunity.
-
-
-
Cardiovascular biology
Macrophages preserve endothelial cell specialization in the adrenal gland to modulate aldosterone secretion and blood pressure.
In Cell Rep on 23 July 2024 by Fan, Z., Karakone, M., et al.
PubMed
Macrophages play crucial roles in organ-specific functions and homeostasis. In the adrenal gland, macrophages closely associate with sinusoidal capillaries in the aldosterone-producing zona glomerulosa. We demonstrate that macrophages preserve capillary specialization and modulate aldosterone secretion. Using macrophage-specific deletion of VEGF-A, single-cell transcriptomics, and functional phenotyping, we found that the loss of VEGF-A depletes PLVAP+ fenestrated endothelial cells in the zona glomerulosa, leading to increased basement membrane collagen IV deposition and subendothelial fibrosis. This results in increased aldosterone secretion, called "haptosecretagogue" signaling. Human aldosterone-producing adenomas also show capillary rarefaction and basement membrane thickening. Mice with myeloid cell-specific VEGF-A deletion exhibit elevated serum aldosterone, hypokalemia, and hypertension, mimicking primary aldosteronism. These findings underscore macrophage-to-endothelial cell signaling as essential for endothelial cell specialization, adrenal gland function, and blood pressure regulation, with broader implications for other endocrine organs.
-
-
-
Cancer Research
-
Immunology and Microbiology
An in-situ peptide-antibody self-assembly to block CD47 and CD24 signaling enhances macrophage-mediated phagocytosis and anti-tumor immune responses.
In Nat Commun on 6 July 2024 by Zhang, W., Zeng, Y., et al.
PubMed
Targeted immunomodulation for reactivating innate cells, especially macrophages, holds great promise to complement current adaptive immunotherapy. Nevertheless, there is still a lack of high-performance therapeutics for blocking macrophage phagocytosis checkpoint inhibitors in solid tumors. Herein, a peptide-antibody combo-supramolecular in situ assembled CD47 and CD24 bi-target inhibitor (PAC-SABI) is described, which undergoes biomimetic surface propagation on cancer cell membranes through ligand-receptor binding and enzyme-triggered reactions. By simultaneously blocking CD47 and CD24 signaling, PAC-SABI enhances the phagocytic ability of macrophages in vitro and in vivo, promoting anti-tumor responses in breast and pancreatic cancer mouse models. Moreover, building on the foundation of PAC-SABI-induced macrophage repolarization and increased CD8+ T cell tumor infiltration, sequential anti-PD-1 therapy further suppresses 4T1 tumor progression, prolonging survival rate. The in vivo construction of PAC-SABI-based nano-architectonics provides an efficient platform for bridging innate and adaptive immunity to maximize therapeutic potency.
-
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Metabolic Reprogramming of Tumor-Associated Macrophages Using Glutamine Antagonist JHU083 Drives Tumor Immunity in Myeloid-Rich Prostate and Bladder Cancers.
In Cancer Immunol Res on 2 July 2024 by Praharaj, M., Shen, F., et al.
PubMed
Glutamine metabolism in tumor microenvironments critically regulates antitumor immunity. Using the glutamine-antagonist prodrug JHU083, we report potent tumor growth inhibition in urologic tumors by JHU083-reprogrammed tumor-associated macrophages (TAMs) and tumor-infiltrating monocytes. We show JHU083-mediated glutamine antagonism in tumor microenvironments induced by TNF, proinflammatory, and mTORC1 signaling in intratumoral TAM clusters. JHU083-reprogrammed TAMs also exhibited increased tumor cell phagocytosis and diminished proangiogenic capacities. In vivo inhibition of TAM glutamine consumption resulted in increased glycolysis, a broken tricarboxylic acid (TCA) cycle, and purine metabolism disruption. Although the antitumor effect of glutamine antagonism on tumor-infiltrating T cells was moderate, JHU083 promoted a stem cell-like phenotype in CD8+ T cells and decreased the abundance of regulatory T cells. Finally, JHU083 caused a global shutdown in glutamine-utilizing metabolic pathways in tumor cells, leading to reduced HIF-1α, c-MYC phosphorylation, and induction of tumor cell apoptosis, all key antitumor features. Altogether, our findings demonstrate that targeting glutamine with JHU083 led to suppressed tumor growth as well as reprogramming of immunosuppressive TAMs within prostate and bladder tumors that promoted antitumor immune responses. JHU083 can offer an effective therapeutic benefit for tumor types that are enriched in immunosuppressive TAMs.
-