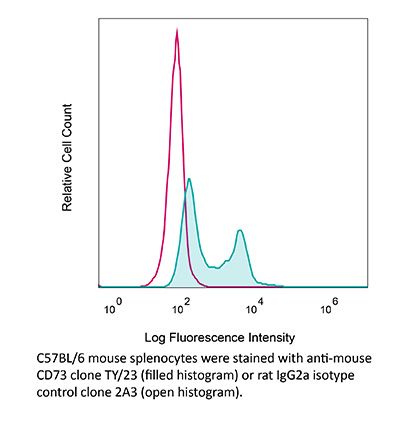
InVivoMAb anti-mouse CD73
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | BALB/c mouse spleen cells and CHO cells transfected with the mouse CD73 gene |
| Reported Applications |
in vivo CD73 blockade in vitro CD73 blockade |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950310 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro CD73 blockade
Zhang, F., et al (2019). "Specific Decrease in B-Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8(+) T Cell Responses" Immunity 50(3): 738-750.e737.
PubMed
Systemic immunosuppression greatly affects the chemotherapeutic antitumor effect. Here, we showed that CD19(+) extracellular vesicles (EVs) from B cells through CD39 and CD73 vesicle-incorporated proteins hydrolyzed ATP from chemotherapy-treated tumor cells into adenosine, thus impairing CD8(+) T cell responses. Serum CD19(+) EVs were increased in tumor-bearing mice and patients. Patients with fewer serum CD19(+) EVs had a better prognosis after chemotherapy. Upregulated hypoxia-inducible factor-1α (HIF-1α) promoted B cells to release CD19(+) EVs by inducing Rab27a mRNA transcription. Rab27a or HIF-1α deficiency in B cells inhibited CD19(+) EV production and improved the chemotherapeutic antitumor effect. Silencing of Rab27a in B cells by inactivated Epstein-Barr viruses carrying Rab27a siRNA greatly improved chemotherapeutic efficacy in humanized immunocompromised NOD Prkdc(scid)Il2rg(-/-) mice. Thus, decreasing CD19(+) EVs holds high potential to improve the chemotherapeutic antitumor effect.
in vivo CD73 blockade
Allard, B., et al (2014). "Anti-CD73 therapy impairs tumor angiogenesis" Int J Cancer 134(6): 1466-1473.
PubMed
CD73 is an ecto-nucleotidase overexpressed in various types of tumors that catabolizes the generation of extracellular adenosine, a potent immunosuppressor. We and others have shown that targeted blockade of CD73 can rescue anti-tumor T cells from the immunosuppressive effects of extracellular adenosine. Another important function of extracellular adenosine is to regulate adaptive responses to hypoxia. However, the importance of CD73 for tumor angiogenesis and the effect of anti-CD73 therapy on tumor angiogenesis remain unknown. In this study, we demonstrated that CD73 expression on tumor cells and host cells contribute to tumor angiogenesis. Our data revealed that tumor-derived CD73 enhances the production of vascular endothelial growth factor (VEGF) by tumor cells that host-derived CD73 is required for in vivo angiogenic responses and that endothelial cells require CD73 expression for tube formation and migration. Notably, the pro-angiogeneic effects of CD73 relied on both enzymatic and non-enzymatic functions. Using a mouse model of breast cancer, we demonstrated that targeted blockade of CD73 with a monoclonal antibody significantly decreased tumor VEGF levels and suppressed tumor angiogenesis in vivo. Taken together, our study strongly suggests that targeted blockade of CD73 can significantly block tumor angiogenesis, and further supports its clinical development for cancer treatment.
in vivo CD73 blockade
Allard, B., et al (2013). "Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs" Clin Cancer Res 19(20): 5626-5635.
PubMed
PURPOSE: Monoclonal antibodies (mAb) that block programmed death (PD)-1 or cytotoxic T lymphocyte antigen (CTLA-4) receptors have been associated with durable clinical responses against a variety of cancer types and hold great potential as novel cancer therapeutics. Recent evidence suggest that targeted blockade of multiple immunosuppressive pathways can induce synergistic antitumor responses. EXPERIMENTAL DESIGN: In this study, we investigated whether targeted blockade of CD73, an ectonucleotidase that catabolizes the hydrolysis of extracellular adenosine monophosphate (AMP) to adenosine, can enhance the antitumor activity of anti-CTLA-4 and anti-PD-1 mAbs against transplanted and chemically induced mouse tumors. RESULTS: Anti-CD73 mAb significantly enhanced the activity of both anti-CTLA-4 and anti-PD-1 mAbs against MC38-OVA (colon) and RM-1 (prostate) subcutaneous tumors, and established metastatic 4T1.2 breast cancer. Anti-CD73 mAb also significantly enhanced the activity of anti-PD-1 mAb against 3-methylcholanthrene (MCA)-induced fibrosarcomas. Gene-targeted mice revealed that single-agent therapies and combinatorial treatments were dependent on host IFN-gamma and CD8(+) T cells, but independent of perforin. Interestingly, anti-CD73 mAb preferentially synergized with anti-PD-1 mAb. We investigated the effect of extracellular adenosine on tumor-infiltrating T cells and showed that activation of A2A adenosine receptor enhances PD-1 expression, but not CTLA-4 expression, on tumor-specific CD8+ T cells and CD4+ Foxp3+ T regulatory cells. CONCLUSIONS: Taken together, our study revealed that targeted blockade of CD73 can enhance the therapeutic activity of anti-PD-1 and anti-CTLA-4 mAbs and may thus potentiate therapeutic strategies targeting immune checkpoint inhibitors in general.
Product Citations
-
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Flow cytometry/Cell sorting
Metabolic reprograming mediated by tumor cell-intrinsic type I IFN signaling is required for CD47-SIRPα blockade efficacy.
In Nat Commun on 9 July 2024 by Zhou, H., Wang, W., et al.
PubMed
Type I interferons have been well recognized for their roles in various types of immune cells during tumor immunotherapy. However, their direct effects on tumor cells are less understood. Oxidative phosphorylation is typically latent in tumor cells. Whether oxidative phosphorylation can be targeted for immunotherapy remains unclear. Here, we find that tumor cell responsiveness to type I, but not type II interferons, is essential for CD47-SIRPα blockade immunotherapy in female mice. Mechanistically, type I interferons directly reprogram tumor cell metabolism by activating oxidative phosphorylation for ATP production in an ISG15-dependent manner. ATP extracellular release is also promoted by type I interferons due to enhanced secretory autophagy. Functionally, tumor cells with genetic deficiency in oxidative phosphorylation or autophagy are resistant to CD47-SIRPα blockade. ATP released upon CD47-SIRPα blockade is required for antitumor T cell response induction via P2X7 receptor-mediated dendritic cell activation. Based on this mechanism, combinations with inhibitors of ATP-degrading ectoenzymes, CD39 and CD73, are designed and show synergistic antitumor effects with CD47-SIRPα blockade. Together, these data reveal an important role of type I interferons on tumor cell metabolic reprograming for tumor immunotherapy and provide rational strategies harnessing this mechanism for enhanced efficacy of CD47-SIRPα blockade.
-
-
-
Immunology and Microbiology
-
Genetics
CD8+ T cells sustain antitumor response by mediating crosstalk between adenosine A2A receptor and glutathione/GPX4.
In J Clin Invest on 5 March 2024 by Chen, S., Fan, J., et al.
PubMed
Antitumor responses of CD8+ T cells are tightly regulated by distinct metabolic fitness. High levels of glutathione (GSH) are observed in the majority of tumors, contributing to cancer progression and treatment resistance in part by preventing glutathione peroxidase 4-dependent (GPX4-dependent) ferroptosis. Here, we show the necessity of adenosine A2A receptor (A2AR) signaling and the GSH/GPX4 axis in orchestrating metabolic fitness and survival of functionally competent CD8+ T cells. Activated CD8+ T cells treated ex vivo with simultaneous inhibition of A2AR and lipid peroxidation acquire a superior capacity to proliferate and persist in vivo, demonstrating a translatable means to prevent ferroptosis in adoptive cell therapy. Additionally, we identify a particular cluster of intratumoral CD8+ T cells expressing a putative gene signature of GSH metabolism (GMGS) in association with clinical response and survival across several human cancers. Our study addresses a key role of GSH/GPX4 and adenosinergic pathways in fine-tuning the metabolic fitness of antitumor CD8+ T cells.
-
-
-
Stem Cells and Developmental Biology
synNotch-programmed iPSC-derived NK cells usurp TIGIT and CD73 activities for glioblastoma therapy.
In Nat Commun on 1 March 2024 by Lupo, K. B., Yao, X., et al.
PubMed
Severe heterogeneity within glioblastoma has spurred the notion that disrupting the interplay between multiple elements on immunosuppression is at the core of meaningful anti-tumor responses. T cell immunoreceptor with Ig and ITIM domains (TIGIT) and its glioblastoma-associated antigen, CD155, form a highly immunosuppressive axis in glioblastoma and other solid tumors, yet targeting of TIGIT, a functionally heterogeneous receptor on tumor-infiltrating immune cells, has largely been ineffective as monotherapy, suggesting that disruption of its inhibitory network might be necessary for measurable responses. It is within this context that we show that the usurpation of the TIGIT - CD155 axis via engineered synNotch-mediated activation of induced pluripotent stem cell-derived natural killer (NK) cells promotes transcription factor-mediated activation of a downstream signaling cascade that results in the controlled, localized blockade of CD73 to disrupt purinergic activity otherwise resulting in the production and accumulation of immunosuppressive extracellular adenosine. Such "decoy" receptor engages CD155 binding to TIGIT, but tilts inhibitory TIGIT/CD155 interactions toward activation via downstream synNotch signaling. Usurping activities of TIGIT and CD73 promotes the function of adoptively transferred NK cells into intracranial patient-derived models of glioblastoma and enhances their natural cytolytic functions against this tumor to result in complete tumor eradication. In addition, targeting both receptors, in turn, reprograms the glioblastoma microenvironment via the recruitment of T cells and the downregulation of M2 macrophages. This study demonstrates that TIGIT/CD155 and CD73 are targetable receptor partners in glioblastoma. Our data show that synNotch-engineered pluripotent stem cell-derived NK cells are not only effective mediators of anti-glioblastoma responses within the setting of CD73 and TIGIT/CD155 co-targeting, but represent a powerful allogeneic treatment option for this tumor.
-
-
-
Immunology and Microbiology
-
Genetics
-
Cancer Research
Dual role of CD73 as a signaling molecule and adenosine-generating enzyme in colorectal cancer progression and immune evasion.
In Int J Biol Sci on 2 January 2024 by Lian, W., Jiang, D., et al.
PubMed
Metastasis and limited benefits of immune checkpoint blockade are two obstacles to the battle against colorectal cancer (CRC). CD73, encoded by the gene 5'-Nucleotidase Ecto (NT5E), is a major enzyme that generates extracellular adenosine. However, whether CD73 affects cancer progression and immune response in CRC remains unclear. Here, the clinical significance of CD73 was assessed in human CRC specimens using immunohistochemistry and bioinformatic analyses. We demonstrated that CD73 is elevated in CRC tissues, particularly in those with metastasis, and correlates with poor prognosis. Gain- and loss-of-function experiments demonstrate that tumor CD73 supports tumor progression and impairs the viability and effector functions of CD8+ T cells. Targeting CD73 on CRC cells reduces their malignant phenotypes and improves the anti-cancer response of CD8+ T cells in the tumor microenvironment (TME). Moreover, the combination of CD73 blockade and PD-1 inhibitors exhibited enhanced anti-cancer effects when compared to a single-agent treatment. Thus, CD73 may be a promising target in the treatment of CRC.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cardiovascular biology
-
Immunology and Microbiology
Nt5e deficiency does not affect post-stroke inflammation and lesion size in a murine ischemia/reperfusion stroke model.
In iScience on 17 June 2022 by Schädlich, I. S., Schnapauff, O., et al.
PubMed
Extracellular ATP released to the ischemic brain parenchyma is quickly metabolized by ectonucleotidases. Among them, the ecto-5'-nucleotidase CD73 encoded by Nt5e generates immunosuppressive adenosine. Genetic deletion of Nt5e led to increased infarct size in the murine photothrombotic stroke model. We aimed at validating this result using the transient middle cerebral artery occlusion (tMCAO) stroke model that represents pathophysiological aspects of penumbra and reperfusion. Three days after tMACO, we did not detect a difference in stroke size between CD73-deficient (CD73-/-) and control mice. Consistent with this finding, CD73-/- and control mice showed comparable numbers and composition of brain-infiltrating leukocytes measured by flow cytometry. Using NanoString technology, we further demonstrated that CD73-/- and control mice do not differ regarding glia cell gene expression profiles. Our findings highlight the potential impact of stroke models on study outcome and the need for cross-validation of originally promising immunomodulatory candidates.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
CD73 induces GM-CSF/MDSC-mediated suppression of T cells to accelerate pancreatic cancer pathogenesis.
In Oncogene on 1 February 2022 by King, R. J., Shukla, S. K., et al.
PubMed
Metabolic alterations regulate cancer aggressiveness and immune responses. Given the poor response of pancreatic ductal adenocarcinoma (PDAC) to conventional immunotherapies, we investigated the link between metabolic alterations and immunosuppression. Our metabolic enzyme screen indicated that elevated expression of CD73, an ecto-5'-nucleotidase that generates adenosine, correlates with increased aggressiveness. Correspondingly, we observed increased interstitial adenosine levels in tumors from spontaneous PDAC mouse models. Diminishing CD73 by genetic manipulations ablated in vivo tumor growth, and decreased myeloid-derived suppressor cells (MDSC) in orthotopic mouse models of PDAC. A high-throughput cytokine profiling demonstrated decreased GM-CSF in mice implanted with CD73 knockdowns. Furthermore, we noted increased IFN-γ expression by intratumoral CD4+ and CD8+ T cells in pancreatic tumors with CD73 knockdowns. Depletion of CD4+ T cells, but not CD8+ T cells abrogated the beneficial effects of decreased CD73. We also observed that splenic MDSCs from Nt5e knockdown tumor-bearing mice were incompetent in suppressing T cell activation in the ex vivo assays. Replenishing GM-CSF restored tumor growth in Nt5e knockout tumors, which was reverted by MDSC depletion. Finally, anti-CD73 antibody treatment significantly improved gemcitabine efficacy in orthotopic models. Thus, targeting the adenosine axis presents a novel therapeutic opportunity for improving the anti-tumoral immune response against PDAC.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
Elimination of acquired resistance to PD-1 blockade via the concurrent depletion of tumour cells and immunosuppressive cells.
In Nat Biomed Eng on 1 November 2021 by Xue, G., Wang, Z., et al.
PubMed
Antigen release resulting from the death of tumour cells induced by chemotherapies and targeted therapies can augment the antitumour responses induced by immune checkpoint blockade (ICB). However, tumours responding to ICB therapies often become resistant to them. Here we show that the specific targeting of tumour cells promotes the growth of tumour-cell variants that are resistant to ICB, and that the acquired resistance can be overcome via the concurrent depletion of tumour cells and of major types of immunosuppressive cell via a monoclonal antibody binding the enzyme CD73, which we identified as highly expressed on tumour cells and on regulatory T cells, myeloid-derived suppressor cells and tumour-associated macrophages, but not on cytolytic T lymphocytes, natural killer cells and dendritic cells. In mice with murine tumours, the systemic administration of anti-PD1 antibodies and anti-CD73 antibodies conjugated to a near-infrared dye prevented near-infrared-irradiated tumours from acquiring resistance to ICB and resulted in the eradication of advanced tumours. The elimination of immunosuppressive cells may overcome acquired resistance to ICB across a range of tumour types and combination therapies.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Profiling of patients with glioma reveals the dominant immunosuppressive axis is refractory to immune function restoration.
In JCI Insight on 3 September 2020 by Ott, M., Tomaszowski, K. H., et al.
PubMed
In order to prioritize available immune therapeutics, immune profiling across glioma grades was conducted, followed by preclinical determinations of therapeutic effect in immune-competent mice harboring gliomas. T cells and myeloid cells were isolated from the blood of healthy donors and the blood and tumors from patients with glioma and profiled for the expression of immunomodulatory targets with an available therapeutic. Murine glioma models were used to assess therapeutic efficacy of agents targeting the most frequently expressed immune targets. In patients with glioma, the A2aR/CD73/CD39 pathway was most frequently expressed, followed by the PD-1 pathway. CD73 expression was upregulated on immune cells by 2-hydroxyglutarate in IDH1 mutant glioma patients. In murine glioma models, adenosine receptor inhibitors demonstrated a modest therapeutic response; however, the addition of other inhibitors of the adenosine pathway did not further enhance this therapeutic effect. Although adenosine receptor inhibitors could recover immunological effector functions in T cells, immune recovery was impaired in the presence of gliomas, indicating that irreversible immune exhaustion limits the effectiveness of adenosine pathway inhibitors in patients with glioma. This study illustrates vetting steps that should be considered before clinical trial implementation for immunotherapy-resistant cancers, including testing an agent's ability to restore immunological function in the context of intended use.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
CD73 Blockade Promotes Dendritic Cell Infiltration of Irradiated Tumors and Tumor Rejection.
In Cancer Immunol Res on 1 April 2020 by Wennerberg, E., Spada, S., et al.
PubMed
The ability of focal radiotherapy to promote priming of tumor-specific CD8+ T cells and increase responses to immunotherapy is dependent on infiltration of the tumor by Batf3-dependent conventional dendritic cell type 1 (cDC1) cells. Such infiltration is driven by radiotherapy-induced IFN type I (IFN-I). Other signals may also modulate cDC1 infiltration of irradiated tumors. Here we found increased expression of adenosine-generating enzymes CD38 and CD73 in irradiated mouse and human breast cancer cells and increased adenosine in mouse tumors following radiotherapy. CD73 blockade alone had no effect. CD73 blockade with radiotherapy restored radiotherapy-induced cDC1 infiltration of tumors in settings where radiotherapy induction of IFN-I was suboptimal. In the absence of radiotherapy-induced IFN-I, blockade of CD73 was required for rejection of the irradiated tumor and for systemic tumor control (abscopal effect) in the context of cytotoxic T-lymphocyte-associated protein 4 blockade. These results suggest that CD73 may be a radiation-induced checkpoint, and that CD73 blockade in combination with radiotherapy and immune checkpoint blockade might improve patient response to therapy.
-
-
-
In vivo experiments
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Specific Decrease in B-Cell-Derived Extracellular Vesicles Enhances Post-Chemotherapeutic CD8+ T Cell Responses.
In Immunity on 19 March 2019 by Zhang, F., Li, R., et al.
PubMed
Systemic immunosuppression greatly affects the chemotherapeutic antitumor effect. Here, we showed that CD19+ extracellular vesicles (EVs) from B cells through CD39 and CD73 vesicle-incorporated proteins hydrolyzed ATP from chemotherapy-treated tumor cells into adenosine, thus impairing CD8+ T cell responses. Serum CD19+ EVs were increased in tumor-bearing mice and patients. Patients with fewer serum CD19+ EVs had a better prognosis after chemotherapy. Upregulated hypoxia-inducible factor-1α (HIF-1α) promoted B cells to release CD19+ EVs by inducing Rab27a mRNA transcription. Rab27a or HIF-1α deficiency in B cells inhibited CD19+ EV production and improved the chemotherapeutic antitumor effect. Silencing of Rab27a in B cells by inactivated Epstein-Barr viruses carrying Rab27a siRNA greatly improved chemotherapeutic efficacy in humanized immunocompromised NOD PrkdcscidIl2rg-/- mice. Thus, decreasing CD19+ EVs holds high potential to improve the chemotherapeutic antitumor effect.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
-
In vivo experiments
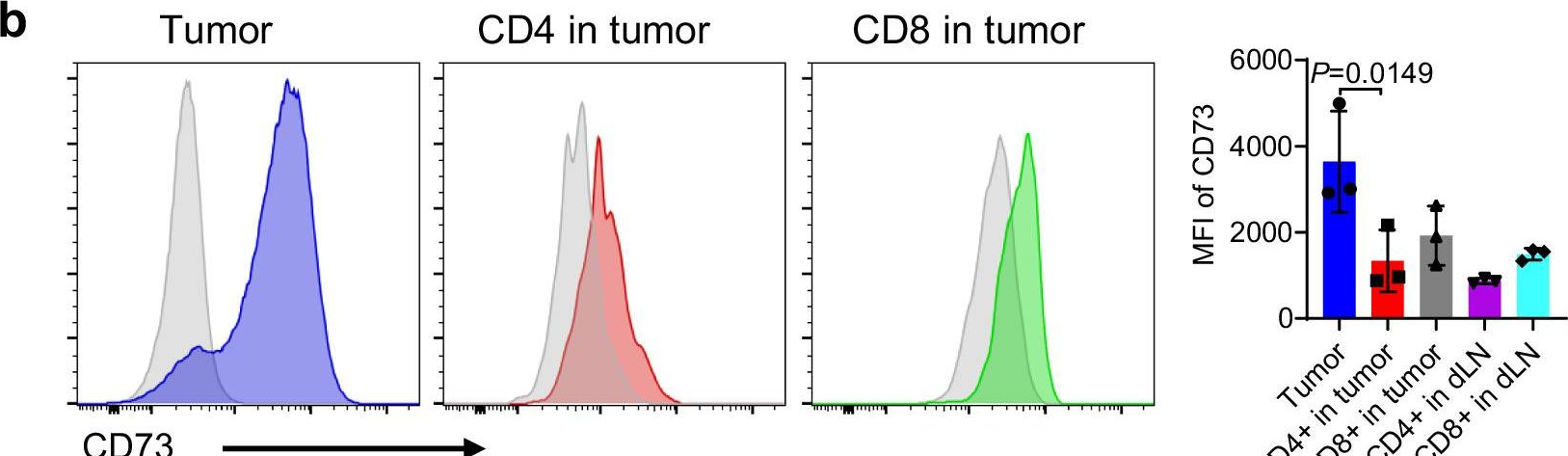
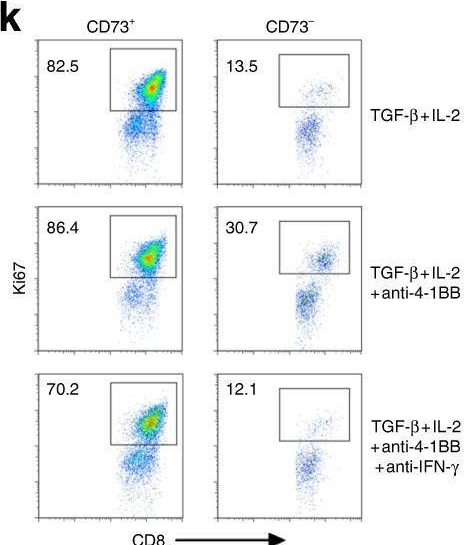
CD73 expression on effector T cells sustained by TGF-β facilitates tumor resistance to anti-4-1BB/CD137 therapy.
In Nat Commun on 11 January 2019 by Chen, S., Fan, J., et al.
PubMed
Agonist antibodies (Ab) directed against costimulatory molecules on the surface of antigen-primed T cells are in various stages of pre-clinical and clinical trials, albeit with limited therapeutic benefit as single agents. The underlying mechanisms of action remain incompletely understood. Here, we demonstrate an inhibitory role of ecto-enzyme CD73 for agonistic anti-4-1BB/CD137 Ab therapy. In particular, anti-4-1BB treatment preferentially drives CD73- effector T cell response for tumor inhibition. Anti-CD73 neutralizing Ab further improves anti-4-1BB therapy associated with enhanced anti-tumor T cell immunity. However, the TGF-β-rich tumor milieu confers resistance to anti-4-1BB therapy by sustaining CD73 expression primarily on infiltrating CD8+ T cells across several tumor models. TGF-β blockade results in downregulation of CD73 expression on infiltrating T cells and sensitizes resistant tumors to agonistic anti-4-1BB therapy. Thus, our findings identify a mechanism of action for more effective clinical targeting of 4-1BB or likely other costimulatory molecules.
-
-
-
Cancer Research
-
Immunology and Microbiology
Specific blockade CD73 alters the exhausted phenotype of T cells in head and neck squamous cell carcinoma.
In Int J Cancer on 15 September 2018 by Deng, W. W., Li, Y. C., et al.
PubMed
The adenosine-induced immunosuppression hampers the immune response toward tumor cells and facilitates the tumor cells to evade immunosurveillance. CD73, an ecto-5-nucleotidase, is the ectoenzyme dephosphorylating extracellular AMP to adenosine. Here, using immunocompetent transgenic head and neck squamous cell carcinoma (HNSCC) mouse model, immune profiling showed high expression of CD73 on CD4+ and CD8+ T cells was associated with an "exhausted" phenotype. Further, treatment with anti-CD73 monoclonal antibody (mAb) significantly blunted the tumor growth in the mouse model, and the blockade of CD73 reversed the "exhausted" phenotype of CD4+ and CD8+ T cells through downregulation of total expression of PD-1 and CTLA-4 on T cells. Whereas the population of CD4+ CD73hi /CD8+ CD73hi T cells expressed higher CTLA-4 and PD-1 as compared to untreated controls. In addition, the human tissue microarrays showed the expression of CD73 is upregulated on tumor infiltrating immune cells in patients with primary HNSCC. Moreover, CD73 expression is an independent prognostic factor for poor outcome in our cohort of HNSCC patients. Altogether, these findings highlight the immunoregulatory role of CD73 in the development of HNSCC and we propose that CD73 may prove to be a promising immunotherapeutic target for the treatment of HNSCC.
-
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses.
In Cancer Cell on 12 September 2016 by Young, A., Ngiow, S. F., et al.
PubMed
Preclinical studies targeting the adenosinergic pathway have gained much attention for their clinical potential in overcoming tumor-induced immunosuppression. Here, we have identified that co-blockade of the ectonucleotidase that generates adenosine CD73 and the A2A adenosine receptor (A2AR) that mediates adenosine signaling in leuokocytes, by using compound gene-targeted mice or therapeutics that target these molecules, limits tumor initiation, growth, and metastasis. This tumor control requires effector lymphocytes and interferon-γ, while antibodies targeting CD73 promote an optimal therapeutic response in vivo when engaging activating Fc receptors. In a two-way mixed leukocyte reaction using a fully human anti-CD73, we demonstrated that Fc receptor binding augmented the production of proinflammatory cytokines.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
Anti-CD73 therapy impairs tumor angiogenesis.
In Int J Cancer on 15 March 2014 by Allard, B., Turcotte, M., et al.
PubMed
CD73 is an ecto-nucleotidase overexpressed in various types of tumors that catabolizes the generation of extracellular adenosine, a potent immunosuppressor. We and others have shown that targeted blockade of CD73 can rescue anti-tumor T cells from the immunosuppressive effects of extracellular adenosine. Another important function of extracellular adenosine is to regulate adaptive responses to hypoxia. However, the importance of CD73 for tumor angiogenesis and the effect of anti-CD73 therapy on tumor angiogenesis remain unknown. In this study, we demonstrated that CD73 expression on tumor cells and host cells contribute to tumor angiogenesis. Our data revealed that tumor-derived CD73 enhances the production of vascular endothelial growth factor (VEGF) by tumor cells that host-derived CD73 is required for in vivo angiogenic responses and that endothelial cells require CD73 expression for tube formation and migration. Notably, the pro-angiogeneic effects of CD73 relied on both enzymatic and non-enzymatic functions. Using a mouse model of breast cancer, we demonstrated that targeted blockade of CD73 with a monoclonal antibody significantly decreased tumor VEGF levels and suppressed tumor angiogenesis in vivo. Taken together, our study strongly suggests that targeted blockade of CD73 can significantly block tumor angiogenesis, and further supports its clinical development for cancer treatment.
-