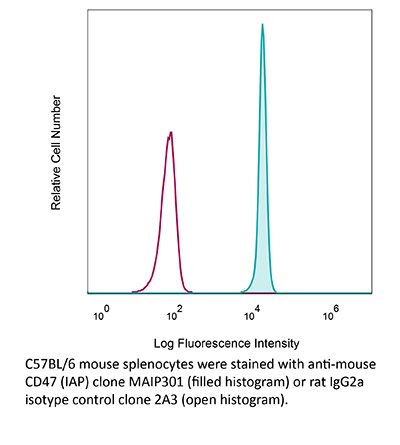
InVivoMAb anti-mouse CD47 (IAP)
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Intact CD47 purified from placenta |
| Reported Applications |
in vivo CD47 blockade in vitro CD47 blockade Immunofluorescence |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687793 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vitro CD47 blockade
Liu, M., et al (2019). "Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated ‘don’t-eat-me’ signal" Nat Immunol 20(3): 265-275.
PubMed
Macrophages enforce antitumor immunity by engulfing and killing tumor cells. Although these functions are determined by a balance of stimulatory and inhibitory signals, the role of macrophage metabolism is unknown. Here, we study the capacity of macrophages to circumvent inhibitory activity mediated by CD47 on cancer cells. We show that stimulation with a CpG oligodeoxynucleotide, a Toll-like receptor 9 agonist, evokes changes in the central carbon metabolism of macrophages that enable antitumor activity, including engulfment of CD47(+) cancer cells. CpG activation engenders a metabolic state that requires fatty acid oxidation and shunting of tricarboxylic acid cycle intermediates for de novo lipid biosynthesis. This integration of metabolic inputs is underpinned by carnitine palmitoyltransferase 1A and adenosine tri-phosphate citrate lyase, which, together, impart macrophages with antitumor potential capable of overcoming inhibitory CD47 on cancer cells. Our findings identify central carbon metabolism to be a novel determinant and potential therapeutic target for stimulating antitumor activity by macrophages.
in vivo CD47 blockade
Pan, Y., et al (2019). "Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer" J Hematol Oncol 12(1): 124.
PubMed
BACKGROUND: Human pancreatic ductal adenocarcinoma (PDAC) responds poorly to immune checkpoint inhibitor (ICPi). While the mechanism is not completely clear, it has been recognized that tumor microenvironment (TME) plays key roles. We investigated if targeting CD47 with a monoclonal antibody could enhance the response of PDAC to ICPi by altering the TME. METHODS: Using immunohistochemistry, we examined tumor-infiltrating CD68(+) pan-macrophages (CD68(+) M) and CD163(+) M2 macrophages (CD163(+) M2) and tumor expression of CD47 and PD-L1 proteins in 106 cases of PDAC. The efficacy of CD47 blockade was examined in xenograft models. CD45(+) immune cells from syngeneic tumor models were subjected to single-cell RNA-sequencing (scRNA-seq) by using the 10x Genomics pipeline. RESULTS: We found that CD47 expression correlated with the level of CD68(+) M but not CD163(+) M2. High levels of tumor-infiltrating CD68(+) M, CD163(+) M2, and CD47 expression were significantly associated with worse survival. CD47(high)/CD68(+) M(high) and CD47(high)/CD163(+) M2(high) correlated significantly with shorter survival, whereas CD47(low)/CD68(+) M(low) and CD47(low)/CD163(+) M2(low) correlated with longer survival. Intriguingly, CD47 blockade decreased the tumor burden in the Panc02 but not in the MPC-83 syngeneic mouse model. Using scRNA-seq, we showed that anti-CD47 treatment significantly remodeled the intratumoral lymphocyte and macrophage compartments in Panc02 tumor-bearing mice by increasing the pro-inflammatory macrophages that exhibit anti-tumor function, while reducing the anti-inflammatory macrophages. Moreover, CD47 blockade not only increased the number of intratumoral CD8(+) T cells, but also remodeled the T cell cluster toward a more activated one. Further, combination therapy targeting both CD47 and PD-L1 resulted in synergistic inhibition of PDAC growth in the MPC-83 but not in Panc02 model. MPC-83 but not Panc02 mice treated with both anti-CD47 and anti-PD-L1 showed increased number of PD-1(+)CD8(+) T cells and enhanced expression of key immune activating genes. CONCLUSION: Our data indicate that CD47 targeting induces compartmental remodeling of tumor-infiltrating immune cells of the TME in PDAC. Different PDAC mouse models exhibited differential response to the anti-CD47 and anti-PD-L1 blockade due to the differential effect of this combination treatment on the infiltrating immune cells and key immune activating genes in the TME established by the different PDAC cell lines.
in vivo CD47 blockade
in vitro CD47 blockade
Reed, M., et al (2019). "Epithelial CD47 is critical for mucosal repair in the murine intestine in vivo" Nat Commun 10(1): 5004.
PubMed
CD47 is a ubiquitously expressed transmembrane glycoprotein that regulates inflammatory responses and tissue repair. Here, we show that normal mice treated with anti-CD47 antibodies, and Cd47-null mice have impaired intestinal mucosal wound healing. Furthermore, intestinal epithelial cell (IEC)-specific loss of CD47 does not induce spontaneous immune-mediated intestinal barrier disruption but results in defective mucosal repair after biopsy-induced colonic wounding or Dextran Sulfate Sodium (DSS)-induced mucosal damage. In vitro analyses using primary cultures of CD47-deficient murine colonic IEC or human colonoid-derived IEC treated with CD47-blocking antibodies demonstrate impaired epithelial cell migration in wound healing assays. Defective wound repair after CD47 loss is linked to decreased epithelial β1 integrin and focal adhesion signaling, as well as reduced thrombospondin-1 and TGF-β1. These results demonstrate a critical role for IEC-expressed CD47 in regulating mucosal repair and raise important considerations for possible alterations in wound healing secondary to therapeutic targeting of CD47.
in vivo CD47 blockade
Sallets, A., et al (2018). "Enhancing immunotherapy of STING agonist for lymphoma in preclinical models" Blood Adv 2(17): 2230-2241.
PubMed
Direct activation of tumor infiltrating antigen-presenting cells (APCs) by intratumoral injection of STING agonists (STINGa) leads to regression of the treated lymphoma tumor. Because STING activation induces apoptosis in lymphoma cells in vitro, we distinguished between the direct therapeutic vs the indirect immunotherapeutic properties of STINGa in vivo. Employing wild-type or STING knockout hosts bearing either wild-type or STING knockout tumor cells, we demonstrated that local tumor regression is totally dependent on STING expression by the host and is therefore immune mediated. However, distant untreated tumors are weakly affected after injection of STINGa to a single tumor site. Therefore, using the STINGa currently being tested in clinical trials, we screened for immunomodulatory agents that could synergize with the STING pathway to induce a systemic antitumor immune response and regression of distant tumors. We combined the STINGa with agents that improve APC or T-cell function. We found that modulation of both APCs and T cells can enhance control of distant lymphoma tumors by STINGa. In particular, adding an anti-GITR antibody induced lymphocyte expansion in the lymph node draining the treated site followed by increased T-cell infiltration in the distant tumor. Furthermore, more of these CD8 T cells at the distant site expressed PD-1. Therefore, blockade of PD-1 further enhanced tumor control at the distant site, leading to cure in 50% of the mice. These preclinical data provide the rationale for testing local injection of STINGa followed by agonistic anti-GITR and anti-PD-1 antibodies as immunotherapy for human lymphoma.
in vivo CD47 blockade
Wu, L., et al (2018). "Anti-CD47 treatment enhances anti-tumor T-cell immunity and improves immunosuppressive environment in head and neck squamous cell carcinoma" Oncoimmunology 7(4): e1397248.
PubMed
Head and neck squamous cell carcinoma (HNSCC) is considered as an immunosuppressive disease, with impaired tumor-infiltrating T lymphocytes and increased suppressive immune cells. The efficacy of CD47 antibodies in immune checkpoint therapy is not clearly understood in HNSCC. In this study, human tissue microarrays and immunocompetent transgenic mouse models were used to explore the expression of CD47 and the use of CD47 antibodies in HNSCC. We identified overexpression of CD47 in HNSCC as compared with the control normal human tissue and also in HNSCC mouse models. The expression of CD47 also correlated with clinicopathological parameters as well as outcome. Furthermore, inhibition of CD47 delayed tumor growth and improved tumor microenvironment by stimulating effector T cells and decreasing suppressive immune cells and regulating the function of CD11b(+) Ly6G(+) MDSC. Our data suggest that CD47 blockade may be a potential immunotherapeutic target in human HNSCC.
in vivo CD47 blockade
Xu, M. M., et al (2017). "Dendritic Cells but Not Macrophages Sense Tumor Mitochondrial DNA for Cross-priming through Signal Regulatory Protein alpha Signaling" Immunity 47(2): 363-373 e365.
PubMed
Inhibition of cytosolic DNA sensing represents a strategy that tumor cells use for immune evasion, but the underlying mechanisms are unclear. Here we have shown that CD47-signal regulatory protein alpha (SIRPalpha) axis dictates the fate of ingested DNA in DCs for immune evasion. Although macrophages were more potent in uptaking tumor DNA, increase of DNA sensing by blocking the interaction of SIRPalpha with CD47 preferentially occurred in dendritic cells (DCs) but not in macrophages. Mechanistically, CD47 blockade enabled the activation of NADPH oxidase NOX2 in DCs, which in turn inhibited phagosomal acidification and reduced the degradation of tumor mitochondrial DNA (mtDNA) in DCs. mtDNA was recognized by cyclic-GMP-AMP synthase (cGAS) in the DC cytosol, contributing to type I interferon (IFN) production and antitumor adaptive immunity. Thus, our findings have demonstrated how tumor cells inhibit innate sensing in DCs and suggested that the CD47-SIRPalpha axis is critical for DC-driven antitumor immunity.
in vivo CD47 blockade
Liu, X., et al (2015). "CD47 blockade triggers T cell-mediated destruction of immunogenic tumors" Nat Med 21(10): 1209-1215.
PubMed
Macrophage phagocytosis of tumor cells mediated by CD47-specific blocking antibodies has been proposed to be the major effector mechanism in xenograft models. Here, using syngeneic immunocompetent mouse tumor models, we reveal that the therapeutic effects of CD47 blockade depend on dendritic cell but not macrophage cross-priming of T cell responses. The therapeutic effects of anti-CD47 antibody therapy were abrogated in T cell-deficient mice. In addition, the antitumor effects of CD47 blockade required expression of the cytosolic DNA sensor STING, but neither MyD88 nor TRIF, in CD11c(+) cells, suggesting that cytosolic sensing of DNA from tumor cells is enhanced by anti-CD47 treatment, further bridging the innate and adaptive responses. Notably, the timing of administration of standard chemotherapy markedly impacted the induction of antitumor T cell responses by CD47 blockade. Together, our findings indicate that CD47 blockade drives T cell-mediated elimination of immunogenic tumors.
in vitro CD47 blockade
Immunofluorescence
Hsieh, C. P., et al (2015). "Deficits in cerebellar granule cell development and social interactions in CD47 knockout mice" Dev Neurobiol 75(5): 463-484.
PubMed
CD47 is involved in neurite differentiation in cultured neurons, but the function of CD47 in brain development is largely unknown. We determined that CD47 mRNA was robustly expressed in the developing cerebellum, especially in granule cells. CD47 protein was mainly expressed in the inner layer of the external granule layer (EGL), molecular layer, and internal granule layer (IGL), where granule cells individually become postmitotic and migrate, leading to neurite fasciculation. At postnatal day 8 (P8), CD47 knockout mice exhibited an increased number of proliferating granule cells in the EGL, whereas the CD47 agonist peptide 4N1K increased the number of postmitotic cells in primary granule cells. Knocking out the CD47 gene and anti-CD47 antibody impaired the radial migration of granule cells from the EGL to the IGL individually in mice and slice cultures. In primary granule cells, knocking out CD47 reduced the number of axonal collaterals and dendritic branches; by contrast, overexpressing CD47 or 4N1K treatment increased the axonal length and numbers of axonal collaterals and dendritic branches. Furthermore, the length of the fissure between Lobules VI and VII was decreased in CD47 knockout mice at P21 and at 14 wk after birth. Lastly, CD47 knockout mice exhibited increased social interaction at P21 and depressive-like behaviors at 10 wk after birth. Our study revealed that the cell adhesion molecule CD47 participates in multiple phases of granule cell development, including proliferation, migration, and neurite differentiation implying that aberrations of CD47 are risk factors that cause abnormalities in cerebellar development and atypical behaviors.
in vivo CD47 blockade
Maute, R. L., et al (2015). "Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging" Proc Natl Acad Sci U S A 112(47): E6506-6514.
PubMed
Signaling through the immune checkpoint programmed cell death protein-1 (PD-1) enables tumor progression by dampening antitumor immune responses. Therapeutic blockade of the signaling axis between PD-1 and its ligand programmed cell death ligand-1 (PD-L1) with monoclonal antibodies has shown remarkable clinical success in the treatment of cancer. However, antibodies have inherent limitations that can curtail their efficacy in this setting, including poor tissue/tumor penetrance and detrimental Fc-effector functions that deplete immune cells. To determine if PD-1:PD-L1-directed immunotherapy could be improved with smaller, nonantibody therapeutics, we used directed evolution by yeast-surface display to engineer the PD-1 ectodomain as a high-affinity (110 pM) competitive antagonist of PD-L1. In contrast to anti-PD-L1 monoclonal antibodies, high-affinity PD-1 demonstrated superior tumor penetration without inducing depletion of peripheral effector T cells. Consistent with these advantages, in syngeneic CT26 tumor models, high-affinity PD-1 was effective in treating both small (50 mm(3)) and large tumors (150 mm(3)), whereas the activity of anti-PD-L1 antibodies was completely abrogated against large tumors. Furthermore, we found that high-affinity PD-1 could be radiolabeled and applied as a PET imaging tracer to efficiently distinguish between PD-L1-positive and PD-L1-negative tumors in living mice, providing an alternative to invasive biopsy and histological analysis. These results thus highlight the favorable pharmacology of small, nonantibody therapeutics for enhanced cancer immunotherapy and immune diagnostics.
in vivo CD47 blockade
Shi, L., et al (2015). "CD47 deficiency ameliorates autoimmune nephritis in Fas(lpr) mice by suppressing IgG autoantibody production" J Pathol 237(3): 285-295.
PubMed
CD47, a self-recognition marker, plays an important role in both innate and adaptive immune responses. To explore the potential role of CD47 in activation of autoreactive T and B cells and the production of autoantibodies in autoimmune disease, especially systemic lupus erythematosus (SLE), we have generated CD47 knockout Fas(lpr) (CD47(-/-) -Fas(lpr) ) mice and examined histopathological changes in the kidneys, cumulative survival rates, proteinuria, extent of splenomegaly and autoantibodies, serum chemistry and immunological parameters. In comparison with Fas(lpr) mice, CD47(-/-) -Fas(lpr) mice exhibit a prolonged lifespan and delayed autoimmune nephritis, including glomerular cell proliferation, basement membrane thickening, acute tubular atrophy and vacuolization. CD47(-/-) -Fas(lpr) mice have lower levels of proteinuria, associated with reduced deposition of complement C3 and C1q, and IgG but not IgM in the glomeruli, compared to age-matched Fas(lpr) mice. Serum levels of antinuclear antibodies and anti-double-stranded DNA antibodies are significantly lower in CD47(-/-) -Fas(lpr) than in Fas(lpr) mice. CD47(-/-) -Fas(lpr) mice also display less pronounced splenomegaly than Fas(lpr) mice. The mechanistic studies further suggest that CD47 deficiency impairs the antigenic challenge-induced production of IgG but not IgM, and that this effect is associated with reduction of T follicular cells and impairment of germinal centre development in lymphoid tissues. In conclusion, our results demonstrate that CD47 deficiency ameliorates lupus nephritis in Fas(lpr) mice via suppression of IgG autoantibody production. Copyright (c) 2015 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
in vitro CD47 blockade
Vermeer, D. W., et al (2013). "Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer" Int J Cancer 133(1): 120-129.
PubMed
The increasing incidence of human papillomavirus (HPV) related oropharyngeal squamous cell carcinoma (OSSC) demands development of novel therapies. Despite presenting at a more advanced stage, HPV(+) oropharyngeal squamous cell carcinoma (OSCC) have a better prognosis than their HPV(-) counterparts. We have previously demonstrated that clearance of HPV(+) OSCC during treatment with radiation and chemotherapy requires an immune response which is likely responsible for the improved clinical outcomes. To further elucidate the mechanism of immune-mediated clearance, we asked whether radiation therapy induces tumor cell changes that allow the body to recognize and aid in tumor clearance. Here, we describe a radiation-induced change in tumor surface protein expression that is critical for immune-mediated clearance. Radiation therapy decreases surface expression of CD47, a self-marker. CD47 is frequently overexpressed in head and neck squamous cell carcinoma and radiation induces a decrease of CD47 in a dose-dependent manner. We show that both in vitro and in vivo tumor cell CD47 protein levels are restored over time after sublethal radiation exposure and that protein levels on adjacent, normal tissues remain unaffected. Furthermore, reduction of tumor cell CD47 increases phagocytosis of these cells by dendritic cells and leads to increased interferon gamma and granzyme production from mixed lymphocytes. Finally, decreasing tumor cell CD47 in combination with standard radiation and chemotherapy results in improved immune-mediated tumor clearance in vivo. These findings help define an important mechanism of radiation-related immune clearance and suggest that decreasing CD47 specifically on tumor cells may be a good therapeutic target for HPV related disease.
in vitro CD47 blockade
Majeti, R., et al (2009). "CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells" Cell 138(2): 286-299.
PubMed
Acute myeloid leukemia (AML) is organized as a cellular hierarchy initiated and maintained by a subset of self-renewing leukemia stem cells (LSC). We hypothesized that increased CD47 expression on human AML LSC contributes to pathogenesis by inhibiting their phagocytosis through the interaction of CD47 with an inhibitory receptor on phagocytes. We found that CD47 was more highly expressed on AML LSC than their normal counterparts, and that increased CD47 expression predicted worse overall survival in three independent cohorts of adult AML patients. Furthermore, blocking monoclonal antibodies directed against CD47 preferentially enabled phagocytosis of AML LSC and inhibited their engraftment in vivo. Finally, treatment of human AML LSC-engrafted mice with anti-CD47 antibody depleted AML and targeted AML LSC. In summary, increased CD47 expression is an independent, poor prognostic factor that can be targeted on human AML stem cells with blocking monoclonal antibodies capable of enabling phagocytosis of LSC.
in vivo CD47 blockade
Chang, H. P., et al (2001). "Functional blocking of integrin-associated protein impairs memory retention and decreases glutamate release from the hippocampus" Neuroscience 102(2): 289-296.
PubMed
We have previously demonstrated that integrin-associated protein is involved in memory consolidation of one-way inhibitory avoidance learning in rats and mice. In the present study, we examined the effects of functional blocking of integrin-associated protein on memory retention, long-term potentiation and glutamate release in mice as well as on cell attachment to extracellular matrix protein in primary cultures. The results indicated that integrin-associated protein monoclonal antibody miap301, when directly injected into the dentate gyrus of the hippocampus at moderate doses, significantly impairs memory retention in mice in the same one-way inhibitory avoidance task and decreases the amplitude of tetanic stimulation-induced long-term potentiation in dentate gyrus neurons. At a dose that effectively impairs both memory retention and long-term potentiation, integrin-associated protein monoclonal antibody also significantly blocks potassium chloride-induced glutamate release from the hippocampus in vivo. Results from western blot confirmed the presence of integrin-associated protein at the synaptic area. Cell adhesion experiments further revealed that integrin-associated protein monoclonal antibody markedly inhibits granular cell attachment to thrombospondin, the extracellular matrix protein known to bind integrin-associated protein, but not to collagen and laminin, the extracellular matrix proteins known to bind integrin. From these results we suggest that integrin-associated protein monoclonal antibody may impair synaptic plasticity and behavioral plasticity in mice through blockade of granular cell attachment to extracellular matrix protein and the subsequent signal transduction, and through inhibition of glutamate release from the hippocampus.
Product Citations
-
-
In Vivo
-
Block
-
Mus musculus (House mouse)
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer.
In Journal of Hematology & Oncology on 27 November 2019 by Pan, Y., Lu, F., et al.
PubMed
Human pancreatic ductal adenocarcinoma (PDAC) responds poorly to immune checkpoint inhibitor (ICPi). While the mechanism is not completely clear, it has been recognized that tumor microenvironment (TME) plays key roles. We investigated if targeting CD47 with a monoclonal antibody could enhance the response of PDAC to ICPi by altering the TME. Using immunohistochemistry, we examined tumor-infiltrating CD68+ pan-macrophages (CD68+ M) and CD163+ M2 macrophages (CD163+ M2) and tumor expression of CD47 and PD-L1 proteins in 106 cases of PDAC. The efficacy of CD47 blockade was examined in xenograft models. CD45+ immune cells from syngeneic tumor models were subjected to single-cell RNA-sequencing (scRNA-seq) by using the 10x Genomics pipeline. We found that CD47 expression correlated with the level of CD68+ M but not CD163+ M2. High levels of tumor-infiltrating CD68+ M, CD163+ M2, and CD47 expression were significantly associated with worse survival. CD47high/CD68+ Mhigh and CD47high/CD163+ M2high correlated significantly with shorter survival, whereas CD47low/CD68+ Mlow and CD47low/CD163+ M2low correlated with longer survival. Intriguingly, CD47 blockade decreased the tumor burden in the Panc02 but not in the MPC-83 syngeneic mouse model. Using scRNA-seq, we showed that anti-CD47 treatment significantly remodeled the intratumoral lymphocyte and macrophage compartments in Panc02 tumor-bearing mice by increasing the pro-inflammatory macrophages that exhibit anti-tumor function, while reducing the anti-inflammatory macrophages. Moreover, CD47 blockade not only increased the number of intratumoral CD8+ T cells, but also remodeled the T cell cluster toward a more activated one. Further, combination therapy targeting both CD47 and PD-L1 resulted in synergistic inhibition of PDAC growth in the MPC-83 but not in Panc02 model. MPC-83 but not Panc02 mice treated with both anti-CD47 and anti-PD-L1 showed increased number of PD-1+CD8+ T cells and enhanced expression of key immune activating genes. Our data indicate that CD47 targeting induces compartmental remodeling of tumor-infiltrating immune cells of the TME in PDAC. Different PDAC mouse models exhibited differential response to the anti-CD47 and anti-PD-L1 blockade due to the differential effect of this combination treatment on the infiltrating immune cells and key immune activating genes in the TME established by the different PDAC cell lines.
-
-
-
In Vitro
-
Block
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Metabolic rewiring of macrophages by CpG potentiates clearance of cancer cells and overcomes tumor-expressed CD47-mediated 'don't-eat-me' signal.
In Nature Immunology on 21 January 2019 by Liu, M., O'Connor, R. S., et al.
PubMed
Macrophages enforce antitumor immunity by engulfing and killing tumor cells. Although these functions are determined by a balance of stimulatory and inhibitory signals, the role of macrophage metabolism is unknown. Here, we study the capacity of macrophages to circumvent inhibitory activity mediated by CD47 on cancer cells. We show that stimulation with a CpG oligodeoxynucleotide, a Toll-like receptor 9 agonist, evokes changes in the central carbon metabolism of macrophages that enable antitumor activity, including engulfment of CD47+ cancer cells. CpG activation engenders a metabolic state that requires fatty acid oxidation and shunting of tricarboxylic acid cycle intermediates for de novo lipid biosynthesis. This integration of metabolic inputs is underpinned by carnitine palmitoyltransferase 1A and adenosine tri-phosphate citrate lyase, which, together, impart macrophages with antitumor potential capable of overcoming inhibitory CD47 on cancer cells. Our findings identify central carbon metabolism to be a novel determinant and potential therapeutic target for stimulating antitumor activity by macrophages.
-
-
-
Cancer Research
-
Immunology and Microbiology
USP2 inhibition unleashes CD47-restrained phagocytosis and enhances anti-tumor immunity.
In Nat Commun on 16 May 2025 by Dai, P., Sun, Y., et al.
PubMed
The CD47/SIRPα axis conveys a 'don't eat me' signal, thereby thwarting the phagocytic clearance of tumor cells. Although blocking antibodies targeting CD47 have demonstrated promising anti-tumor effects in preclinical models, clinical trials involving human cancer patients have not yielded ideal results. Exploring the regulatory mechanisms of CD47 is imperative for devising more efficacious combinational therapies. Here, we report that inhibiting USP2 prompts CD47 degradation and reshapes the tumor microenvironment (TME), thereby enhancing anti-PD-1 immunotherapy. Mechanistically, USP2 interacts with CD47, stabilizing it through deubiquitination. USP2 inhibition destabilizes CD47, thereby boosting macrophage phagocytosis. Single-cell RNA sequencing shows USP2 inhibition reprograms TME, evidenced by increasing M1 macrophages and CD8+ T cells while reducing M2 macrophages. Combining ML364 with anti-PD-1 reduces tumor burden in mouse models. Clinically, low USP2 expression predicts a better response to anti-PD-1 treatment. Our findings uncover the regulatory mechanism of CD47 by USP2 and targeting this axis boosts anti-tumor immunity.
-
-
-
Immunology and Microbiology
Quantifying treatment response to a macrophage-targeted therapy in combination with immune checkpoint inhibitors after exposure to conventional chemotherapy.
In Front Immunol on 13 May 2025 by Bess, S. N., Smart, G. K., et al.
PubMed
Conventional chemotherapeutic agents, such as 5-fluorouracil (5-FU), can exert anti-tumor effects through immunogenic cell death (ICD) induction. Researchers have found hallmarks that quantify ICD (such as the translocation of HMGB1 and calreticulin). Although chemotherapeutic agents can induce ICD, they increase the expression of immune checkpoints, limiting their effectiveness. Studies have emphasized the importance of investigating the heterogeneous responses of cells co-localized in a solid tumor (macrophages, tumor cells, etc.) to ICD induction. However, these studies were performed in vivo, which limits the collection of information on cell-cell interactions due to model complexity.
-
-
CD47-amyloid-β-CD74 signaling triggers adaptive immunosuppression in sepsis.
In EMBO Rep on 1 May 2025 by Feng, Z., Wang, L., et al.
PubMed
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. However, how this dysregulation occurs remains to be elucidated. In this study, we use single-cell RNA sequencing (scRNA-seq) and conventional RNA-seq to analyze the immune landscape of sepsis and observe that adaptive immunity is acutely and strongly suppressed. This systemic immunosuppression occurs not only in the peripheral blood but also in all other immune compartments, including the spleen, lymph nodes, and bone marrow. Clinical data show that these adaptive immunity-related genes may have the potential to be used to distinguish patients with sepsis from those with common infections. CD47 is found to play a pivotal role in this immunosuppression by inducing the production of amyloid-β (Aβ), which interacts with CD74 on B cells, leading to B-cell suppression and subsequent adaptive immunosuppression. Blocking CD47-Aβ signaling significantly reduces organ injury and improves the survival rate of septic mice by restoring phagocytic cell functions and alleviating B-cell suppression and adaptive immunosuppression.
-
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
Blocking the SIRPα-CD47 axis promotes macrophage phagocytosis of exosomes derived from visceral adipose tissue and improves inflammation and metabolism in mice.
In J Biomed Sci on 28 February 2025 by Lin, Y. K., Pan, Y. F., et al.
PubMed
Adipose tissue plays a pivotal role in systemic metabolism and maintaining bodily homeostasis. Exosomes from adipose tissues, known as AT-Exos, are recognized as important messengers in the communication between adipose tissue and other organs. Despite this, the alterations in exosome composition and the functional disparities among depot-specific AT-Exos in obesity remain elusive.
-
-
-
Cancer Research
-
Endocrinology and Physiology
-
Immunology and Microbiology
Caerin 1.1/1.9-mediated antitumor immunity depends on IFNAR-Stat1 signalling of tumour infiltrating macrophage by autocrine IFNα and is enhanced by CD47 blockade.
In Sci Rep on 30 January 2025 by Li, J., Luo, Y., et al.
PubMed
Previously, we demonstrated that natural host-defence peptide caerin 1.1/caerin 1.9 (F1/F3) increases the efficacy of anti-PD-1 and therapeutic vaccine, in a HPV16 + TC-1 tumour model, but the anti-tumor mechanism of F1/F3 is still unclear. In this study, we explored the impact of F1/F3 on the tumor microenvironment in a transplanted B16 melanoma model, and further investigated the mechanism of action of F1/F3 using monoclonal antibodies to deplete relevant cells, gene knockout mice and flow cytometry. We show that F1/F3 is able to inhibit the growth of melanoma B16 tumour cells both in vitro and in vivo. Depletion of macrophages, blockade of IFNα receptor, and Stat1 inhibition each abolishes F1/F3-mediated antitumor responses. Subsequent analysis reveals that F1/F3 increases the tumour infiltration of inflammatory macrophages, upregulates the level of IFNα receptor, and promotes the secretion of IFNα by macrophages. Interestingly, F1/F3 upregulates CD47 level on tumour cells; and blocking CD47 increases F1/F3-mediated antitumor responses. Furthermore, F1/F3 intratumor injection, CD47 blockade, and therapeutic vaccination significantly increases the survival time of B16 tumour-bearing mice. These results indicate that F1/F3 may be effective to improve the efficacy of ICB and therapeutic vaccine-based immunotherapy for human epithelial cancers and warrants consideration for clinical trials.
-
-
-
In vitro experiments
-
Mus musculus (Mouse)
-
Cancer Research
Caerin 1.1 and 1.9 peptides halt B16 melanoma metastatic tumours via expanding cDC1 and reprogramming tumour macrophages.
In J Transl Med on 28 October 2024 by Fu, Q., Luo, Y., et al.
PubMed
Cancer immunotherapy, particularly immune checkpoint inhibitors (ICBs) such as anti-PD-1 antibodies, has revolutionised cancer treatment, although response rates vary among patients. Previous studies have demonstrated that caerin 1.1 and 1.9, host-defence peptides from the Australian tree frog, enhance the effectiveness of anti-PD-1 and therapeutic vaccines in a murine TC-1 model by activating tumour-associated macrophages intratumorally.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
An in-situ peptide-antibody self-assembly to block CD47 and CD24 signaling enhances macrophage-mediated phagocytosis and anti-tumor immune responses.
In Nat Commun on 6 July 2024 by Zhang, W., Zeng, Y., et al.
PubMed
Targeted immunomodulation for reactivating innate cells, especially macrophages, holds great promise to complement current adaptive immunotherapy. Nevertheless, there is still a lack of high-performance therapeutics for blocking macrophage phagocytosis checkpoint inhibitors in solid tumors. Herein, a peptide-antibody combo-supramolecular in situ assembled CD47 and CD24 bi-target inhibitor (PAC-SABI) is described, which undergoes biomimetic surface propagation on cancer cell membranes through ligand-receptor binding and enzyme-triggered reactions. By simultaneously blocking CD47 and CD24 signaling, PAC-SABI enhances the phagocytic ability of macrophages in vitro and in vivo, promoting anti-tumor responses in breast and pancreatic cancer mouse models. Moreover, building on the foundation of PAC-SABI-induced macrophage repolarization and increased CD8+ T cell tumor infiltration, sequential anti-PD-1 therapy further suppresses 4T1 tumor progression, prolonging survival rate. The in vivo construction of PAC-SABI-based nano-architectonics provides an efficient platform for bridging innate and adaptive immunity to maximize therapeutic potency.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
-
-
Chromosomal instability induced in cancer can enhance macrophage-initiated immune responses that include anti-tumor IgG.
In Elife on 28 May 2024 by Hayes, B., Wang, M., et al.
PubMed
Solid tumors generally exhibit chromosome copy number variation, which is typically caused by chromosomal instability (CIN) in mitosis. The resulting aneuploidy can drive evolution and associates with poor prognosis in various cancer types as well as poor response to T-cell checkpoint blockade in melanoma. Macrophages and the SIRPα-CD47 checkpoint are understudied in such contexts. Here, CIN is induced in poorly immunogenic B16F10 mouse melanoma cells using spindle assembly checkpoint MPS1 inhibitors that generate persistent micronuclei and diverse aneuploidy while skewing macrophages toward a tumoricidal 'M1-like' phenotype based on markers and short-term anti-tumor studies. Mice bearing CIN-afflicted tumors with wild-type CD47 levels succumb similar to controls, but long-term survival is maximized by SIRPα blockade on adoptively transferred myeloid cells plus anti-tumor monoclonal IgG. Such cells are the initiating effector cells, and survivors make de novo anti-cancer IgG that not only promote phagocytosis of CD47-null cells but also suppress tumor growth. CIN does not affect the IgG response, but pairing CIN with maximal macrophage anti-cancer activity increases durable cures that possess a vaccination-like response against recurrence.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Airway epithelial CD47 plays a critical role in inducing influenza virus-mediated bacterial super-infection.
In Nat Commun on 30 April 2024 by Moon, S., Han, S., et al.
PubMed
Respiratory viral infection increases host susceptibility to secondary bacterial infections, yet the precise dynamics within airway epithelia remain elusive. Here, we elucidate the pivotal role of CD47 in the airway epithelium during bacterial super-infection. We demonstrated that upon influenza virus infection, CD47 expression was upregulated and localized on the apical surface of ciliated cells within primary human nasal or bronchial epithelial cells. This induced CD47 exposure provided attachment sites for Staphylococcus aureus, thereby compromising the epithelial barrier integrity. Through bacterial adhesion assays and in vitro pull-down assays, we identified fibronectin-binding proteins (FnBP) of S. aureus as a key component that binds to CD47. Furthermore, we found that ciliated cell-specific CD47 deficiency or neutralizing antibody-mediated CD47 inactivation enhanced in vivo survival rates. These findings suggest that interfering with the interaction between airway epithelial CD47 and pathogenic bacterial FnBP holds promise for alleviating the adverse effects of super-infection.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting HDAC6 improves anti-CD47 immunotherapy.
In J Exp Clin Cancer Res on 27 February 2024 by Gracia-Hernandez, M., Yende, A. S., et al.
PubMed
Cancer cells can overexpress CD47, an innate immune checkpoint that prevents phagocytosis upon interaction with signal regulatory protein alpha (SIRPα) expressed in macrophages and other myeloid cells. Several clinical trials have reported that CD47 blockade reduces tumor growth in hematological malignancies. However, CD47 blockade has shown modest results in solid tumors, including melanoma. Our group has demonstrated that histone deacetylase 6 inhibitors (HDAC6is) have immunomodulatory properties, such as controlling macrophage phenotype and inflammatory properties. However, the molecular and cellular mechanisms controlling these processes are not fully understood. In this study, we evaluated the role of HDAC6 in regulating the CD47/SIRPα axis and phagocytosis in macrophages.
-
-
-
Cancer Research
-
Endocrinology and Physiology
-
Immunology and Microbiology
Caerin 1.1/1.9-mediated antitumor immunity depends on IFNAR-Stat1 signalling of tumour infiltrating macrophage by autocrine of IFNα and is enhanced by CD47 blockade
In Research Square on 13 December 2023 by Li, J., Luo, Y., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
IGF2BP3 Enhances the Growth of Hepatocellular Carcinoma Tumors by Regulating the Properties of Macrophages and CD8+ T Cells in the Tumor Microenvironment.
In J Clin Transl Hepatol on 28 November 2023 by Ma, L., Jiang, J., et al.
PubMed
Overexpression of IGF2BP3 is associated with the prognosis of hepatocellular carcinoma (HCC). However, its role in regulating tumor immune microenvironment (TME) is not well characterized. Here, we investigated the effects of IGF2BP3 on macrophages and CD8+ T cells within the TME of HCC.
-
-
-
Cancer Research
-
In vivo experiments
-
Mus musculus (Mouse)
THBS1-producing tumor-infiltrating monocyte-like cells contribute to immunosuppression and metastasis in colorectal cancer.
In Nat Commun on 25 September 2023 by Omatsu, M., Nakanishi, Y., et al.
PubMed
Mesenchymal activation, characterized by dense stromal infiltration of immune and mesenchymal cells, fuels the aggressiveness of colorectal cancers (CRC), driving progression and metastasis. Targetable molecules in the tumor microenvironment (TME) need to be identified to improve the outcome in CRC patients with this aggressive phenotype. This study reports a positive link between high thrombospondin-1 (THBS1) expression and mesenchymal characteristics, immunosuppression, and unfavorable CRC prognosis. Bone marrow-derived monocyte-like cells recruited by CXCL12 are the primary source of THBS1, which contributes to the development of metastasis by inducing cytotoxic T-cell exhaustion and impairing vascularization. Furthermore, in orthotopically generated CRC models in male mice, THBS1 loss in the TME renders tumors partially sensitive to immune checkpoint inhibitors and anti-cancer drugs. Our study establishes THBS1 as a potential biomarker for identifying mesenchymal CRC and as a critical suppressor of antitumor immunity that contributes to the progression of this malignancy with a poor prognosis.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
Cooperative phagocytosis of solid tumours by macrophages triggers durable anti-tumour responses.
In Nat Biomed Eng on 1 September 2023 by Dooling, L. J., Andrechak, J. C., et al.
PubMed
In solid tumours, the abundance of macrophages is typically associated with a poor prognosis. However, macrophage clusters in tumour-cell nests have been associated with survival in some tumour types. Here, by using tumour organoids comprising macrophages and cancer cells opsonized via a monoclonal antibody, we show that highly ordered clusters of macrophages cooperatively phagocytose cancer cells to suppress tumour growth. In mice with poorly immunogenic tumours, the systemic delivery of macrophages with signal-regulatory protein alpha (SIRPα) genetically knocked out or else with blockade of the CD47-SIRPα macrophage checkpoint was combined with the monoclonal antibody and subsequently triggered the production of endogenous tumour-opsonizing immunoglobulin G, substantially increased the survival of the animals and helped confer durable protection from tumour re-challenge and metastasis. Maximizing phagocytic potency by increasing macrophage numbers, by tumour-cell opsonization and by disrupting the phagocytic checkpoint CD47-SIRPα may lead to durable anti-tumour responses in solid cancers.
-
-
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
Chromosomal instability can favor macrophage-mediated immune response and induce a broad, vaccination-like anti-tumor IgG response
In bioRxiv on 4 April 2023 by Hayes, B. H., Wang, M., et al.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
Blockade of CD47 function attenuates restenosis by promoting smooth muscle cell efferocytosis and inhibiting their migration and proliferation.
In J Biol Chem on 1 April 2023 by Govatati, S., Pichavaram, P., et al.
PubMed
Cluster of differentiation 47 (CD47) plays an important role in the pathophysiology of various diseases including atherosclerosis but its role in neointimal hyperplasia which contributes to restenosis has not been studied. Using molecular approaches in combination with a mouse vascular endothelial denudation model, we studied the role of CD47 in injury-induced neointimal hyperplasia. We determined that thrombin-induced CD47 expression both in human aortic smooth muscle cells (HASMCs) and mouse aortic smooth muscle cells. In exploring the mechanisms, we found that the protease-activated receptor 1-Gα protein q/11 (Gαq/11)-phospholipase Cβ3-nuclear factor of activated T cells c1 signaling axis regulates thrombin-induced CD47 expression in HASMCs. Depletion of CD47 levels using its siRNA or interference of its function by its blocking antibody (bAb) blunted thrombin-induced migration and proliferation of HASMCs and mouse aortic smooth muscle cells. In addition, we found that thrombin-induced HASMC migration requires CD47 interaction with integrin β3. On the other hand, thrombin-induced HASMC proliferation was dependent on CD47's role in nuclear export and degradation of cyclin-dependent kinase-interacting protein 1. In addition, suppression of CD47 function by its bAb rescued HASMC efferocytosis from inhibition by thrombin. We also found that vascular injury induces CD47 expression in intimal SMCs and that inhibition of CD47 function by its bAb, while alleviating injury-induced inhibition of SMC efferocytosis, attenuated SMC migration, and proliferation resulting in reduced neointima formation. Thus, these findings reveal a pathological role for CD47 in neointimal hyperplasia.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field.
In Nat Commun on 23 March 2023 by Ma, X., Liang, X., et al.
PubMed
Micro-nano biorobots based on bacteria have demonstrated great potential for tumor diagnosis and treatment. The bacterial gene expression and drug release should be spatiotemporally controlled to avoid drug release in healthy tissues and undesired toxicity. Herein, we describe an alternating magnetic field-manipulated tumor-homing bacteria developed by genetically modifying engineered Escherichia coli with Fe3O4@lipid nanocomposites. After accumulating in orthotopic colon tumors in female mice, the paramagnetic Fe3O4 nanoparticles enable the engineered bacteria to receive and convert magnetic signals into heat, thereby initiating expression of lysis proteins under the control of a heat-sensitive promoter. The engineered bacteria then lyse, releasing its anti-CD47 nanobody cargo, that is pre-expressed and within the bacteria. The robust immunogenicity of bacterial lysate cooperates with anti-CD47 nanobody to activate both innate and adaptive immune responses, generating robust antitumor effects against not only orthotopic colon tumors but also distal tumors in female mice. The magnetically engineered bacteria also enable the constant magnetic field-controlled motion for enhanced tumor targeting and increased therapeutic efficacy. Thus, the gene expression and drug release behavior of tumor-homing bacteria can be spatiotemporally manipulated in vivo by a magnetic field, achieving tumor-specific CD47 blockage and precision tumor immunotherapy.
-
-
-
Immunology and Microbiology
-
Mus musculus (Mouse)
The CIt protocol: A blueprint to potentiate the immunogenicity of immunoproteasome-reprogrammed mesenchymal stromal cells.
In iScience on 22 December 2022 by Bikorimana, J. P., El-Hachem, N., et al.
PubMed
Immunoproteasome-reprogrammed mesenchymal stromal cells (IRMs) can surpass dendritic cells at eliciting tumor-specific immunity. However, the current IRM vaccination regimen remains clinically unsuitable due to the relatively high dose of IRMs needed. Since the administration of a lower IRM dose triggers a feeble anti-tumoral response, we aimed to combine this vaccination regimen with different modalities to fine-tune the potency of the vaccine. In a nutshell, we found that the co-administration of IRMs and interleukin-12 accentuates the anti-tumoral response, whereas the cross-presentation potency of IRMs is enhanced via intracellular succinate build-up, delayed endosomal maturation, and increased endosome-to-cytosol plasticity. Stimulating phagocyte-mediated cancer efferocytosis by blocking the CD47-SIRPα axis was also found to enhance IRM vaccine outcomes. Upon designing a single protocol combining the abovementioned strategies, 60% of treated animals exhibited a complete response. Altogether, this is the first IRM-based vaccination study, optimized to simultaneously target three vaccine-related pitfalls: T-cell response, antigen cross-presentation, and cancer phagocytosis.
-