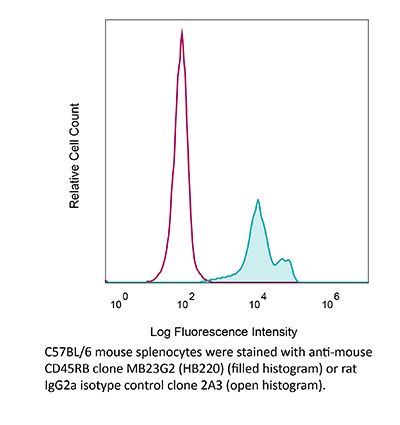
InVivoMAb anti-mouse CD45RB
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Anti-immunoglobulin activated mouse B cells |
| Reported Applications |
in vivo anti-CD45RB_mediated tolerance induction in vivo pre-mNK cell depletion |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107653 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo pre-mNK cell depletion
Wilson, K. A., et al (2015). "Depletion of B220NK1.1 cells enhances the rejection of established melanoma by tumor-specific CD4 T cells" Oncoimmunology 4(8): e1019196.
PubMed
Five-year survival rates for patients diagnosed with metastatic melanoma are less than 5%. Adoptive cell transfer (ACT) has achieved an objective response of 50% by Response Evaluation Criteria in Solid Tumors (RECIST) in this patient population. For ACT to be maximally effective, the host must first be lymphodepleted. It is hypothesized that lymphodepletion may remove regulatory elements and cytokine sinks, or increase the activation and availability of antigen presenting cells (APCs). We use an in vivo model to study the ACT of tumor-associated antigen (TAA)-specific CD4+ T cells (TRP-1 cells). We have discovered that depletion of NK1.1+ cells enhances the rejection of established melanoma tumors by adoptively transferred TRP-1 CD4+ T cells. NK1.1+ cell depletion increases the number of CD4+ T cells, the serum concentration of pro-inflammatory cytokines, autoimmune vitiligo, host survival and prevented recurrence after ACT. Because multiple cells express NK1.1, we targeted different NK1.1+ cell populations using antibodies specific for NK cells, pre-mNK cells, and innate lymphoid cells (ILCs). Our data suggests that NK1.1+B220+ pre-mNK cells (also known as interferon-producing killer dendritic cells; IKDCs) are an important inhibitor of the CD4+ T cell response to melanoma. Understanding this mechanism may help design new immunotherapies to modulate the activity of pre-mNKs in the face of an antitumor immune response and inhibit their suppression of adoptively transferred T cells.
in vivo anti-CD45RB–mediated tolerance induction
Stocks, B. T., et al (2015). "Lupus-Prone Mice Resist Immune Regulation and Transplant Tolerance Induction" Am J Transplant .
PubMed
The strongly immunogenic environment in autoimmune diseases such as lupus may pose a stringent barrier to transplantation. Despite available murine models of lupus, transplant tolerance in this setting has yet to be fully investigated in highly penetrant genetic models of disease. Such studies are of clear clinical importance because lupus is a transplant indication in which transplanted kidneys have a substantially increased risk of rejection including a role for recurrent nephritis. In the fully penetrant B6.SLE123 mouse, we determined that CD4 T follicular helper and germinal center B cells were significantly expanded compared with healthy controls. We traced this expansion to resistance of effector CD4 T and B cells in B6.SLE123 mice to regulation by either CD4 T regulatory cells (CD4Tregs) or CD8 T regulatory cells (CD8Tregs), despite demonstrating normal function by Tregs in this strain. Finally, we determined that B6.SLE123 mice resist anti-CD45RB-mediated tolerance induction to foreign islet allografts, even in the absence of islet autoimmunity. Overall, B6.SLE123 lupus-prone mice are highly resistant to transplant tolerance induction, which provides a new model of failed tolerance in autoimmunity that may elucidate barriers to clinical transplantation in lupus through further cellular and genetic dissection.
in vivo anti-CD45RB–mediated tolerance induction
Lee, K. M., et al (2014). "TGF-beta-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance" Eur J Immunol 44(6): 1728-1736.
PubMed
Regulatory B (Breg) cells have been shown to play a critical role in immune homeostasis and in autoimmunity models. We have recently demonstrated that combined anti-T cell immunoglobulin domain and mucin domain-1 and anti-CD45RB antibody treatment results in tolerance to full MHC-mismatched islet allografts in mice by generating Breg cells that are necessary for tolerance. Breg cells are antigen-specific and are capable of transferring tolerance to untreated, transplanted animals. Here, we demonstrate that adoptively transferred Breg cells require the presence of regulatory T (Treg) cells to establish tolerance, and that adoptive transfer of Breg cells increases the number of Treg cells. Interaction with Breg cells in vivo induces significantly more Foxp3 expression in CD4(+) CD25(-) T cells than with naive B cells. We also show that Breg cells express the TGF-beta associated latency-associated peptide and that Breg-cell mediated graft prolongation post-adoptive transfer is abrogated by neutralization of TGF-beta activity. Breg cells, like Treg cells, demonstrate preferential expression of both C-C chemokine receptor 6 and CXCR3. Collectively, these findings suggest that in this model of antibody-induced transplantation tolerance, Breg cells promote graft survival by promoting Treg-cell development, possibly via TGF-beta production.
in vivo anti-CD45RB–mediated tolerance induction
Kim, J. I., et al (2013). "Elevated levels of interferon-gamma production by memory T cells do not promote transplant tolerance resistance in aged recipients" PLoS One 8(12): e82856.
PubMed
Immunosenescence predisposes the elderly to infectious and autoimmune diseases and impairs the response to vaccination. We recently demonstrated that ageing also impedes development of transplantation tolerance. Unlike their young counterparts (8-12 weeks of age) aged male recipients (greater than 12 months of age) transplanted with a full MHC-mismatched heart are resistant to tolerance mediated by anti-CD45RB antibody. Surprisingly, either chemical or surgical castration restored tolerance induction to levels observed using young recipients. Based on the strong impact of endocrine modulation on transplant tolerance, we explored the impact of ageing and castration on the immune system. Here we report a significant increase in the percentage of T cells that produce interferon-gamma (IFN-gamma) in aged male versus young male animals and that the overall increase in IFN-gamma production was due to an expansion of IFN-gamma-producing memory T cells in aged animals. In contrast to IFN-gamma production, we did not observe differences in IL-10 expression in young versus old male mice. We hypothesized that endocrine modulation would diminish the elevated levels of IFN-gamma production in aged recipients, however, we observed no significant reduction in the percentage of IFN-gamma+ T cells upon castration. Furthermore, we neutralized interferon-gamma by antibody and did not observe an effect on graft survival. We conclude that while elevated levels of interferon-gamma serves as a marker of tolerance resistance in aged mice, other as yet to be identified factors are responsible for its cause. Defining these factors may be relevant to design of tolerogenic strategies for aged recipients.
in vivo anti-CD45RB–mediated tolerance induction
Ge, W., et al (2010). "Regulatory T cells are critical to tolerance induction in presensitized mouse transplant recipients through targeting memory T cells" Am J Transplant 10(8): 1760-1773.
PubMed
Memory T cells are a significant barrier to induction of transplant tolerance. However, reliable means to target alloreactive memory T cells have remained elusive. In this study, presensitization of BALB/c mice with C57BL/6 skin grafts generated a large number of OX40(+)CD44(hi)effector/memory T cells and resulted in rapid rejection of donor heart allografts. Recognizing that anti-OX40L monoclonal antibody (mAb) (alpha-OX40L) monotherapy prolonged graft survival through inhibition and apoptosis of memory T cells in presensitized recipients, alpha-OX40L was added to the combined treatment protocol of LF15-0195 (LF) and anti-CD45RB (alpha-CD45RB) mAb-a protocol that induced heart allograft tolerance in non-presensitized recipients but failed to induce tolerance in presensitized recipients. Interestingly, this triple therapy restored donor-specific heart allograft tolerance in our presensitized model that was associated with induction of tolerogenic dendritic cells and CD4(+)CD25(+)Foxp3(+) T regulatory cells (Tregs). Of note, CD25(+) T cell depletion in triple therapy recipients prevented establishment of allograft tolerance. In addition, adoptive transfer of donor-primed effector/memory T cells into tolerant recipients markedly reduced levels of Tregs and broke tolerance. Our findings indicated that targeting memory T cells, by blocking OX40 costimulation in presensitized recipients was very important to expansion of Tregs, which proved critical to development of tolerance.
in vivo anti-CD45RB–mediated tolerance induction
Shen, H. and D. R. Goldstein (2009). "IL-6 and TNF-alpha synergistically inhibit allograft acceptance" J Am Soc Nephrol 20(5): 1032-1040.
PubMed
Previous studies suggested that activation of the innate immune system impairs the induction of transplantation tolerance, but the responsible inflammatory mediators have not been identified. In this study, we examined whether IL-6 and TNF-alpha promote resistance to transplantation tolerance. Using a highly immunogenic murine skin allograft model, we found that the absence of both IL-6 and TNF-alpha in the graft recipient synergized with co-stimulatory blockade to induce tolerance. Furthermore, IL-6 and TNF-alpha acted together to promote T cell alloimmune responses both in vitro and in vivo and to impair the ability of regulatory T cells to suppress effector T cell alloimmunity. In addition, deficiency of recipient IRAK-M, a negative regulator of certain innate immune pathways, augmented cellular IL-6 and TNF-alpha responses and impaired the ability of co-stimulatory blockade to extend allograft survival. In summary, IL-6 and TNF-alpha synergistically impair the efficacy of therapies that promote allograft acceptance.
Product Citations
-
-
Immunology and Microbiology
Protocol for renal subcapsular islet transplantation in diabetic mice to induce long-term immune tolerance.
In STAR Protoc on 20 June 2025 by Yuan, Y., Wu, Q., et al.
PubMed
Murine renal subcapsular islet transplantation presents a promising technique for diabetes treatment by addressing challenges such as immune rejection and reliance on immunosuppressive drugs. Here, we present a protocol for the isolation, purification, and transplantation of mouse pancreatic islets that overcomes these challenges. Specifically, we describe steps for inducing diabetes with streptozotocin, pancreatic perfusion and isolation, and islet cell purification. We then detail procedures for renal subcapsular islet transplantation, dual antibody therapy, and immune cell and graft monitoring. For complete details on the use and execution of this protocol, please refer to Liu et al.1.
-
-
Versatile tissue-injectable hydrogels capable of the extended hydrolytic release of bioactive protein therapeutics.
In Bioeng Transl Med on 1 September 2024 by Nealy, E. S., Reed, J., et al.
PubMed
Hydrogels are extensively employed in healthcare due to their adaptable structures, high water content, and biocompatibility, with FDA-approved applications ranging from spinal cord regeneration to local therapeutic delivery. However, clinical hydrogels encounter challenges related to inconsistent therapeutic exposure, unmodifiable release windows, and difficulties in subsurface polymer insertion. Addressing these issues, we engineered injectable, biocompatible hydrogels as a local therapeutic depot, utilizing poly(ethylene glycol) (PEG)-based hydrogels functionalized with bioorthogonal SPAAC handles for network polymerization and functionalization. Our hydrogel solutions polymerize in situ in a temperature-sensitive manner, persist in tissue, and facilitate the delivery of bioactive therapeutics in subsurface locations. Demonstrating the efficacy of our approach, recombinant anti-CD47 monoclonal antibodies, when incorporated into subsurface-injected hydrogel solutions, exhibited cytotoxic activity against infiltrative high-grade glioma xenografts in the rodent brain. To enhance the gel's versatility, recombinant protein cargos can undergo site-specific modification with hydrolysable "azidoester" adapters, allowing for user-defined release profiles from the hydrogel. Hydrogel-generated gradients of murine CXCL10, linked to intratumorally injected hydrogel solutions via azidoester linkers, resulted in significant recruitment of CD8+ T-cells and the attenuation of tumor growth in a "cold" syngeneic melanoma model. This study highlights a highly customizable, hydrogel-based delivery system for local protein therapeutic administration to meet diverse clinical needs.
-
A novel prevascularized tissue-engineered chamber as a site for allogeneic and xenogeneic islet transplantation to establish a bioartificial pancreas.
In PLoS One on 4 December 2020 by Liu, Y., Yang, M., et al.
PubMed
Although sites for clinical or experimental islet transplantation are well established, pancreatic islet survival and function in these locations remain unsatisfactory. A possible factor that might account for this outcome is local hypoxia caused by the limited blood supply. Here, we modified a prevascularized tissue-engineered chamber (TEC) that facilitated the viability and function of the seeded islets in vivo by providing a microvascular network prior to transplantation. TECs were created, filled with Growth Factor-Matrigel™ (Matrigel™) and then implanted into the groins of mice with streptozotocin-induced diabetes. The degree of microvascularization in each TECs was analyzed by histology, real-time PCR, and Western blotting. Three hundred syngeneic islets were seeded into each chamber on days 0, 14, and 28 post-chamber implantation, and 300, 200, or 100 syngeneic islets were seeded into additional chambers on day 28 post-implantation, respectively. Furthermore, allogeneic or xenogeneic islet transplantation is a potential solution for organ shortage. The feasibility of TECs as transplantation sites for islet allografts or xenografts and treatment with anti-CD45RB and/or anti-CD40L (MR-1) was therefore explored. A highly developed microvascularized network was established in each TEC on day 28 post-implantation. Normalization of blood glucose levels in diabetic mice was negatively correlated with the duration of prevascularization and the number of seeded syngeneic islets. Combined treatment with anti-CD45RB and MR-1 resulted in long-term survival of the grafts following allotransplantation (5/5, 100%) and xenotransplantation (16/20, 80%). Flow cytometry demonstrated that the frequency of CD4+Foxp3-Treg and CD4+IL-4+-Th2 cells increased significantly after tolerogenic xenograft transplantation, while the number of CD4+IFN-γ-Th1 cells decreased. These findings demonstrate that highly developed microvascularized constructs can facilitate the survival of transplanted islets in a TECs, implying its potential application as artificial pancreas in the future.
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity.
In Nat Commun on 20 March 2020 by von Roemeling, C. A., Wang, Y., et al.
PubMed
Tumour cell phagocytosis by antigen presenting cells (APCs) is critical to the generation of antitumour immunity. However, cancer cells can evade phagocytosis by upregulating anti-phagocytosis molecule CD47. Here, we show that CD47 blockade alone is inefficient in stimulating glioma cell phagocytosis. However, combining CD47 blockade with temozolomide results in a significant pro-phagocytosis effect due to the latter's ability to induce endoplasmic reticulum stress response. Increased tumour cell phagocytosis subsequently enhances antigen cross-presentation and activation of cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) in APCs, resulting in more efficient T cell priming. This bridging of innate and adaptive responses inhibits glioma growth, but also activates immune checkpoint. Sequential administration of an anti-PD1 antibody overcomes this potential adaptive resistance. Together, these findings reveal a dynamic relationship between innate and adaptive immune regulation in tumours and support further investigation of phagocytosis modulation as a strategy to enhance cancer immunotherapy responses.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
-
Pharmacology
Macrophage activation by a substituted pyrimido[5,4-b]indole increases anti-cancer activity.
In Pharmacol Res on 1 October 2019 by Hardie, J., Mas-Rosario, J. A., et al.
PubMed
Immunotherapy has become a promising new approach for cancer treatment due to the immune system's ability to remove tumors in a safe and specific manner. Many tumors express anti-inflammatory factors that deactivate the local immune response or recruit peripheral macrophages into pro-tumor roles. Because of this, effective and specific ways of activating macrophages into anti-tumor phenotypes is highly desirable for immunotherapy purposes. Here, the use of a small molecule TLR agonist as a macrophage activator for anti-cancer therapy is reported. This compound, referred to as PBI1, demonstrated unique activation characteristics and expression patterns compared to treatment with LPS, through activation of TLR4. Furthermore, PBI1 treatment resulted in anti-tumor immune behavior, enhancing macrophage phagocytic efficiency five-fold versus non-treated macrophages. Additive effects were observed via use of a complementary strategy (anti-CD47 antibody), resulting in ∼10-fold enhancement of phagocytosis, suggesting this small molecule approach could be used in conjunction with other therapeutics.
-
-
-
Cancer Research
Characterization of cluster of differentiation 47 expression and its potential as a therapeutic target in esophageal squamous cell cancer.
In Oncol Lett on 1 February 2018 by Zhao, C. L., Yu, S., et al.
PubMed
The increased expression of cluster of differentiation (CD)47 has been identified in a number of different tumor types and is recognized as an adverse prognostic factor that indicates an increased risk of mortality in patients. The binding of CD47 to signal regulatory protein α (SIRPα) inhibits the macrophage phagocytosis of tumor cells by triggering an inhibitory 'do not eat me' signal. This is one of the mechanisms used by tumor cells to evade immune surveillance. In the present study, CD47 levels and macrophage infiltration were assessed in patients with esophageal squamous cell cancer (ESCC). CD47-overexpressing ESCC cell lines were selected and human M2 macrophage phagocytic activity was measured. The results revealed that CD47 is highly expressed and macrophages are markedly infiltrated in cancerous tissue compared with non-cancerous tissue. High CD47 expression was detected in ESCC cell lines and the results of a phagocytosis assay indicated that human M2 macrophages phagocytized tumor cells in a dose-dependent manner following the blocking of CD47-SIRPα signaling by anti-CD47 antibodies. The results of the present study therefore support the use of anti-CD47 immunotherapy to treat patients with ESCC.
-