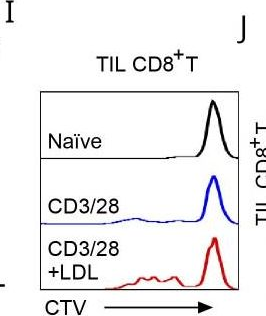
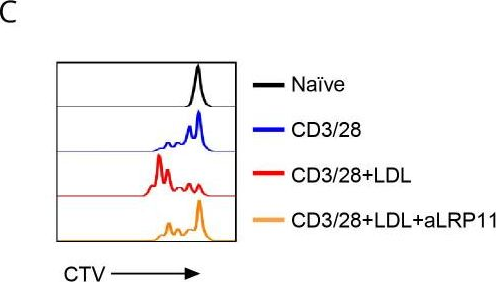
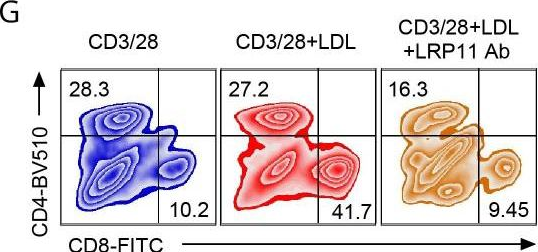
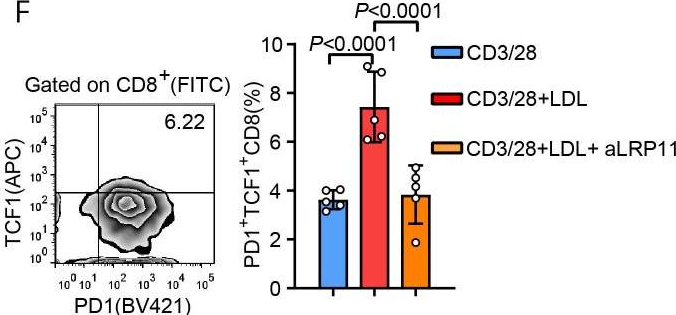
InVivoMAb anti-mouse CD3ε F(ab')2 fragment
Product Description
Specifications
| Isotype | Armenian Hamster IgG1 |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb hamster IgG f(ab')2 fragments |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Immunogen | Mouse BM10-37 cytotoxic T cells |
| Reported Applications | in vivo T cell depletion |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 μM filtered |
| Production | Pepsin Digest |
| Purification | Protein A |
| RRID | AB_2687679 |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo T cell depletion
Sarikonda, G., et al (2015). "The Hsp60 peptide p277 enhances anti-CD3 mediated diabetes remission in non-obese diabetic mice" J Autoimmun 59: 61-66.
PubMed
Type 1 diabetes (T1D) is characterized by the immune-mediated destruction of pancreatic beta cells leading to inadequate glycemic control. Trials with immunomodulatory monotherapies have shown that the disease course can in principle be altered. The observed preservation of endogenous insulin secretion however is typically transient and chronic treatment is often associated with significant side effects. Here we combined anti-CD3 with the Hsp60 peptide p277, two drugs that have been evaluated in Phase 3 trials, to test for enhanced efficacy. Female NOD mice with recent onset diabetes were given 5 mug anti-CD3 i.v., on three consecutive days in combination with 100 mug of p277 peptide in IFA s.c., once weekly for four weeks. Anti-CD3 alone restored normoglycemia in 44% of the mice while combination therapy with anti-CD3 and p277 induced stable remission in 83% of mice. The observed increase in protection occurred only in part through TLR2 signaling and was characterized by increased Treg numbers and decreased insulitis. These results have important implications for the design of combination therapies for the treatment of T1D.
in vivo T cell depletion
Kasahara, K., et al (2014). "CD3 antibody and IL-2 complex combination therapy inhibits atherosclerosis by augmenting a regulatory immune response" J Am Heart Assoc 3(2): e000719.
PubMed
BACKGROUND: Accumulating evidence suggests that the balance between pathogenic effector T cells (Teffs) and regulatory T cells (Tregs) may be important for controlling atherosclerotic disease. We hypothesized that a combination therapy with anti-CD3 antibody (CD3-Ab) and IL-2/anti-IL-2 monoclonal antibody complex (IL-2 complex) aimed at increasing the ratio of Tregs to Teffs would effectively inhibit atherosclerosis in mice. METHODS AND RESULTS: We treated apolipoprotein E-deficient mice fed a high-cholesterol diet with vehicle, CD3-Ab, IL-2 complex, or their combination. Mice receiving the combination therapy had markedly reduced atherosclerotic lesions than mice treated with CD3-Ab or IL-2 complex alone. In addition, a striking increase in the Treg/Teff ratio of lymphoid organs and atherosclerotic lesions, along with plaque stabilization characterized by decreased macrophage content and increased collagen content was observed. The combination treatment also markedly reduced splenic Ly6C(high) inflammatory monocytes and might induce a favorable macrophage phenotype change in atherosclerotic lesions. CONCLUSIONS: Our results indicate that in addition to suppressing Teff responses, enhancing Treg-mediated immune responses is more efficacious in preventing atherosclerosis, suggesting a novel therapeutic approach for atherosclerosis.
in vivo T cell depletion
Kita, T., et al (2014). "Regression of atherosclerosis with anti-CD3 antibody via augmenting a regulatory T-cell response in mice" Cardiovasc Res 102(1): 107-117.
PubMed
AIMS: Although recent animal studies have investigated the cellular and molecular mechanisms underlying the process of atherosclerosis regression, it remains unknown whether adaptive immune responses including T cells are involved in this process. We investigated the role of T cells in atherosclerosis regression. METHODS AND RESULTS: LDL receptor-deficient mice were fed a high-cholesterol diet for 8 weeks to form atherosclerotic lesions and were then changed to a standard diet, and atherosclerosis was assessed 4 weeks later. Just before changing the diet, the mice received an iv injection of anti-CD3 antibody (CD3-Ab) or control immunoglobulin G for 5 consecutive days. CD3-Ab treatment regressed atherosclerosis and decreased the accumulation of macrophages and CD4(+) T cells in the plaques. CD3-Ab treatment also dramatically reduced CD4(+) T cells and increased the proportion of regulatory T cells (Tregs). Depletion of Tregs by anti-CD25 antibody injection abolished the regression of atherosclerosis seen in CD3-Ab-treated mice, indicating the essential role for Tregs in this process. CONCLUSION: CD3-Ab treatment induced rapid regression of established atherosclerosis via reducing CD4(+) T cells and increasing the proportion of Tregs. These findings suggest that therapeutic intervention for T-cell-mediated immune responses may represent a novel strategy to induce atherosclerosis regression in combination with lipid-lowering therapy.
in vivo T cell depletion
Shiheido, H., et al (2014). "Novel CD3-specific antibody induces immunosuppression via impaired phosphorylation of LAT and PLCgamma1 following T-cell stimulation" Eur J Immunol 44(6): 1770-1780.
PubMed
The activation of T cells is known to be accompanied by the temporary downmodulation of the TCR/CD3 complex on the cell surface. Here, we established a novel monoclonal antibody, Dow2, that temporarily induces downmodulation of the TCR/CD3 complex in mouse CD4(+) T cells without activating T cells. Dow2 recognized the determinant on CD3epsilon; however, differences were observed in the binding mode between Dow2 and the agonistic anti-CD3epsilon Ab, 145-2C11. An injection of Dow2 in vivo resulted in T-cell anergy, and prolonged the survival of cardiac allografts without a marked increase in cytokine release. The phosphorylated forms of the signaling proteins PLC-gamma1 and LAT in Dow2-induced anergic T cells were markedly decreased upon stimulation. However, the levels of phosphorylated LAT and PLCgamma1 in Dow2-induced anergic T cells could be rescued in the presence of the proteasome inhibitor MG-132. These results suggest that proteasome-mediated degradation is involved in hypophosphorylated LAT and PLCgamma1 in Dow2-induced anergic T cells. The novel CD3-specific Ab, Dow2, may provide us with a unique tool for inducing immunosuppression.
in vivo T cell depletion
Grant, C. W., et al (2013). "Testing agents for prevention or reversal of type 1 diabetes in rodents" PLoS One 8(8): e72989.
PubMed
We report the results of an independent laboratory’s tests of novel agents to prevent or reverse type 1 diabetes (T1D) in the non-obese diabetic (NOD) mouse, BioBreeding diabetes prone (BBDP) rat, and multiple autoimmune disease prone (MAD) rat models. Methods were developed to better mimic human clinical trials, including: prescreening, randomization, blinding, and improved glycemic care of the animals. Agents were suggested by the research community in an open call for proposals, and selected for testing by an NIDDK appointed independent review panel. Agents selected for testing to prevent diabetes at later stages of progression in a rodent model were a STAT4 antagonist (DT22669), alpha1 anti-trypsin (Aralast NP), celastrol (a natural product with anti-inflammatory properties), and a Macrophage Inflammatory Factor inhibitor (ISO-092). Agents tested for reversal of established T1D in rodent models were: alpha1 anti-trypsin (Aralast NP), tolerogenic peptides (Tregitopes), and a long-acting formulation of GLP-1 (PGC-GLP-1). None of these agents were seen to prevent or reverse type 1 diabetes, while the positive control interventions were effective: anti-CD3 treatment provided disease reversal in the NOD mouse, dexamethasone prevented T1D induction in the MAD rat, and cyclosporin prevented T1D in the BBDP rat. For some tested agents, details of previous formulation, delivery, or dosing, as well as laboratory procedure, availability of reagents and experimental design, could have impacted our ability to confirm prior reports of efficacy in preclinical animal models. In addition, the testing protocols utilized here provided detection of effects in a range commonly used in placebo controlled clinical trials (for example, 50% effect size), and thus may have been underpowered to observe more limited effects. That said, we believe the results compiled here, showing good control and repeatability, confirm the feasibility of screening diverse test agents in an independent laboratory.
Product Citations
-
-
Immunology and Microbiology
Monocyte/macrophage-derived interleukin-15 mediates the pro-inflammatory phenotype of CD226+ B cells in type 1 diabetes.
In EBioMedicine on 1 October 2025 by Li, J., Liang, X., et al.
PubMed
Type 1 diabetes (T1D) is characterised by the autoimmune-mediated destruction of pancreatic β-cells. Although traditionally viewed as a disease dominated by T cells, recent studies have emphasised the crucial role of B cells in the development of T1D. Genome-wide association studies (GWAS) have revealed that CD226 is related to susceptibility to several autoimmune diseases, including T1D. Our recent work identified a pathogenic role of CD226+ CD8+ T cells in T1D. However, the involvement of CD226+ B cells in T1D development remains unclear.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Flow cytometry/Cell sorting
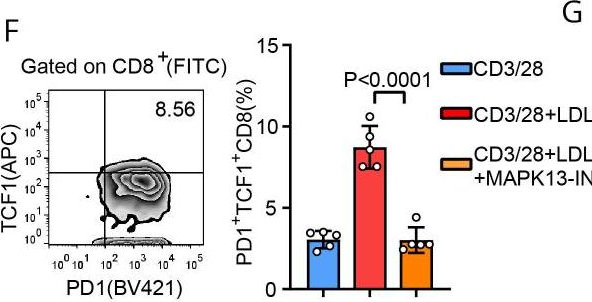
LRP11 promotes stem-like T cells via MAPK13-mediated TCF1 phosphorylation, enhancing anti-PD1 immunotherapy.
In J Immunother Cancer on 25 January 2024 by Sun, L., Ma, Z., et al.
PubMed
Tumor-infiltrating T cells enter an exhausted or dysfunctional state, which limits antitumor immunity. Among exhausted T cells, a subset of cells with features of progenitor or stem-like cells has been identified as TCF1+ CD8+ T cells that respond to immunotherapy. In contrast to the finding that TCF1 controls epigenetic and transcriptional reprogramming in tumor-infiltrating stem-like T cells, little is known about the regulation of TCF1. Emerging data show that elevated body mass index is associated with outcomes of immunotherapy. However, the mechanism has not been clarified.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Cell Biology
-
Biochemistry and Molecular biology
PHGDH-mediated endothelial metabolism drives glioblastoma resistance to chimeric antigen receptor T cell immunotherapy.
In Cell Metab on 7 March 2023 by Zhang, D., Li, A. M., et al.
PubMed
The efficacy of immunotherapy is limited by the paucity of T cells delivered and infiltrated into the tumors through aberrant tumor vasculature. Here, we report that phosphoglycerate dehydrogenase (PHGDH)-mediated endothelial cell (EC) metabolism fuels the formation of a hypoxic and immune-hostile vascular microenvironment, driving glioblastoma (GBM) resistance to chimeric antigen receptor (CAR)-T cell immunotherapy. Our metabolome and transcriptome analyses of human and mouse GBM tumors identify that PHGDH expression and serine metabolism are preferentially altered in tumor ECs. Tumor microenvironmental cues induce ATF4-mediated PHGDH expression in ECs, triggering a redox-dependent mechanism that regulates endothelial glycolysis and leads to EC overgrowth. Genetic PHGDH ablation in ECs prunes over-sprouting vasculature, abrogates intratumoral hypoxia, and improves T cell infiltration into the tumors. PHGDH inhibition activates anti-tumor T cell immunity and sensitizes GBM to CAR T therapy. Thus, reprogramming endothelial metabolism by targeting PHGDH may offer a unique opportunity to improve T cell-based immunotherapy.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
A stable, engineered TL1A ligand co-stimulates T cells via specific binding to DR3.
In Sci Rep on 29 November 2022 by Zwolak, A., Chan, S. R., et al.
PubMed
TL1A (TNFSF15) is a TNF superfamily ligand which can bind the TNFRSF member death receptor 3 (DR3) on T cells and the soluble decoy receptor DcR3. Engagement of DR3 on CD4+ or CD8+ effector T cells by TL1A induces downstream signaling, leading to proliferation and an increase in secretion of inflammatory cytokines. We designed a stable recombinant TL1A molecule that (1) displays high monodispersity and stability, (2) displays the ability to activate T cells in vitro and in vivo, and (3) lacks binding to DcR3 while retaining functional activity via DR3. Together these results suggest the TL1A ligand can be amenable to therapeutic development on its own or paired with a tumor-targeting moiety.
-
-
-
Mus musculus (Mouse)
Ultrasound Imaging of Pancreatic Perfusion Dynamics Predicts Therapeutic Prevention of Diabetes in Preclinical Models of Type 1 Diabetes.
In Ultrasound Med Biol on 1 July 2022 by Pham, V. T., Ciccaglione, M., et al.
PubMed
In type 1 diabetes (T1D), immune-cell infiltration into islets of Langerhans (insulitis) and β-cell decline occur years before diabetes presents. There is a lack of validated clinical approaches for detecting insulitis and β-cell decline, to diagnose eventual diabetes and monitor the efficacy of therapeutic interventions. We previously determined that contrast-enhanced ultrasound measurements of pancreas perfusion dynamics predict disease progression in T1D pre-clinical models. Here, we test whether these measurements predict therapeutic prevention of T1D. We performed destruction-reperfusion measurements with size-isolated microbubbles in non-obese diabetic (NOD)-severe combined immunodeficiency (SCID) mice receiving an adoptive transfer of diabetogenic splenocytes. Mice received vehicle control or the following treatments: (i) anti-CD3 to block T-cell activation; (ii) anti-CD4 to deplete CD4+ T cells; (iii) verapamil to reduce β-cell apoptosis; or (iv) tauroursodeoxycholic acid (TUDCA) to reduce β-cell endoplasmic reticulum stress. We compared measurements of pancreas perfusion dynamics with subsequent progression to diabetes. Anti-CD3, anti-CD4, and verapamil delayed diabetes development. Blood flow dynamics was significantly altered in treated mice with delayed/absent diabetes development compared with untreated mice. Conversely, blood flow dynamics in treated mice with unchanged diabetes development was similar to that in untreated mice. Thus, measurement of pancreas perfusion dynamics predicts the successful prevention of diabetes. This strategy may provide a clinically deployable predictive marker for therapeutic prevention in asymptomatic T1D.
-
-
-
Endocrinology and Physiology
Glucagon-receptor-antagonism-mediated β-cell regeneration as an effective anti-diabetic therapy.
In Cell Rep on 31 May 2022 by Xi, Y., Song, B., et al.
PubMed
Type 1 diabetes mellitus (T1D) is a chronic disease with potentially severe complications, and β-cell deficiency underlies this disease. Despite active research, no therapy to date has been able to induce β-cell regeneration in humans. Here, we discover the β-cell regenerative effects of glucagon receptor antibody (anti-GcgR). Treatment with anti-GcgR in mouse models of β-cell deficiency leads to reversal of hyperglycemia, increase in plasma insulin levels, and restoration of β-cell mass. We demonstrate that both β-cell proliferation and α- to β-cell transdifferentiation contribute to anti-GcgR-induced β-cell regeneration. Interestingly, anti-GcgR-induced α-cell hyperplasia can be uncoupled from β-cell regeneration after antibody clearance from the body. Importantly, we are able to show that anti-GcgR-induced β-cell regeneration is also observed in non-human primates. Furthermore, anti-GcgR and anti-CD3 combination therapy reverses diabetes and increases β-cell mass in a mouse model of autoimmune diabetes.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
Reprogramming NK cells and macrophages via combined antibody and cytokine therapy primes tumors for elimination by checkpoint blockade.
In Cell Rep on 23 November 2021 by Wang, C., Cui, A., et al.
PubMed
Treatments aiming to augment immune checkpoint blockade (ICB) in cancer often focus on T cell immunity, but innate immune cells may have important roles to play. Here, we demonstrate a single-dose combination treatment (termed AIP) using a pan-tumor-targeting antibody surrogate, half-life-extended interleukin-2 (IL-2), and anti-programmed cell death 1 (PD-1), which primes tumors to respond to subsequent ICB and promotes rejection of large established tumors in mice. Natural killer (NK) cells and macrophages activated by AIP treatment underwent transcriptional reprogramming; rapidly killed cancer cells; governed the recruitment of cross-presenting dendritic cells (DCs) and other leukocytes; and induced normalization of the tumor vasculature, facilitating further immune infiltration. Thus, innate cell-activating therapies can initiate critical steps leading to a self-sustaining cycle of T cell priming driven by ICB.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Endocrinology and Physiology
-
Immunology and Microbiology
T cells mediate cell non-autonomous arterial ageing in mice.
In J Physiol on 1 August 2021 by Trott, D. W., Machin, D. R., et al.
PubMed
Increased large artery stiffness and impaired endothelium-dependent dilatation occur with advanced age. We sought to determine whether T cells mechanistically contribute to age-related arterial dysfunction. We found that old mice exhibited greater proinflammatory T cell accumulation around both the aorta and mesenteric arteries. Pharmacologic depletion or genetic deletion of T cells in old mice resulted in ameliorated large artery stiffness and greater endothelium-dependent dilatation compared with mice with T cells intact.
-
-
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Nanoparticle-Mediated Lipid Metabolic Reprogramming of T Cells in Tumor Microenvironments for Immunometabolic Therapy.
In Nanomicro Lett on 4 January 2021 by Kim, D., Wu, Y., et al.
PubMed
aCD3/F/AN, anti-CD3e f(ab')2 fragment-modified and fenofibrate-encapsulated amphiphilic nanoparticle, reprogrammed mitochondrial lipid metabolism of T cells. aCD3/F/AN specifically activated T cells in glucose-deficient conditions mimicking tumor microenvironment, and exerted an effector killing effect against tumor cells. In vivo treatment with aCD3/F/AN increased T cell infiltration, cytokine production, and prevented tumor growth. We report the activation of anticancer effector functions of T cells through nanoparticle-induced lipid metabolic reprogramming. Fenofibrate was encapsulated in amphiphilic polygamma glutamic acid-based nanoparticles (F/ANs), and the surfaces of F/ANs were modified with an anti-CD3e f(ab')2 fragment, yielding aCD3/F/ANs. An in vitro study reveals enhanced delivery of aCD3/F/ANs to T cells compared with plain F/ANs. aCD3/F/AN-treated T cells exhibited clear mitochondrial cristae, a higher membrane potential, and a greater mitochondrial oxygen consumption rate under glucose-deficient conditions compared with T cells treated with other nanoparticle preparations. Peroxisome proliferator-activated receptor-α and downstream fatty acid metabolism-related genes are expressed to a greater extent in aCD3/F/AN-treated T cells. Activation of fatty acid metabolism by aCD3/F/ANs supports the proliferation of T cells in a glucose-deficient environment mimicking the tumor microenvironment. Real-time video recordings show that aCD3/F/AN-treated T cells exerted an effector killing effect against B16F10 melanoma cells. In vivo administration of aCD3/F/ANs can increase infiltration of T cells into tumor tissues. The treatment of tumor-bearing mice with aCD3/F/ANs enhances production of various cytokines in tumor tissues and prevented tumor growth. Our findings suggest the potential of nanotechnology-enabled reprogramming of lipid metabolism in T cells as a new modality of immunometabolic therapy.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Inhibition of IRF4 in dendritic cells by PRR-independent and -dependent signals inhibit Th2 and promote Th17 responses.
In Elife on 4 February 2020 by Lee, J., Zhang, J., et al.
PubMed
Cyclic AMP (cAMP) is involved in many biological processes but little is known regarding its role in shaping immunity. Here we show that cAMP-PKA-CREB signaling (a pattern recognition receptor [PRR]-independent mechanism) regulates conventional type-2 Dendritic Cells (cDC2s) in mice and reprograms their Th17-inducing properties via repression of IRF4 and KLF4, transcription factors essential for cDC2-mediated Th2 induction. In mice, genetic loss of IRF4 phenocopies the effects of cAMP on Th17 induction and restoration of IRF4 prevents the cAMP effect. Moreover, curdlan, a PRR-dependent microbial product, activates CREB and represses IRF4 and KLF4, resulting in a pro-Th17 phenotype of cDC2s. These in vitro and in vivo results define a novel signaling pathway by which cDC2s display plasticity and provide a new molecular basis for the classification of novel cDC2 and cDC17 subsets. The findings also reveal that repressing IRF4 and KLF4 pathway can be harnessed for immuno-regulation.
-
-
-
Immunodepletion
-
Immunodepletion
-
Mus musculus (Mouse)
-
Immunology and Microbiology
c-MYC overexpression induces choroid plexus papillomas through a T-cell mediated inflammatory mechanism.
In Acta Neuropathol Commun on 29 May 2019 by Merve, A., Zhang, X., et al.
PubMed
Choroid plexus tumours (CPTs) account for 2-5% of brain tumours in children. They can spread along the neuraxis and can recur after treatment. Little is known about the molecular mechanisms underlying their formation and only few high fidelity mouse models of p53-deficient malignant CPTs are available.We show here that c-MYC overexpression in the choroid plexus epithelium induces T-cell inflammation-dependent choroid plexus papillomas in a mouse model. We demonstrate that c-MYC is expressed in a substantial proportion of human choroid plexus tumours and that this subgroup of tumours is characterised by an inflammatory transcriptome and significant inflammatory infiltrates. In compound mutant mice, overexpression of c-MYC in an immunodeficient background led to a decreased incidence of CPP and reduced tumour bulk. Finally, reduced tumour size was also observed upon T-cell depletion in CPP-bearing mice. Our data raise the possibility that benign choroid plexus tumours expressing c-MYC could be amenable to medical therapy with anti-inflammatory drugs.
-
-
-
In vivo experiments
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cardiovascular biology
-
Endocrinology and Physiology
Testosterone Protects Against Atherosclerosis in Male Mice by Targeting Thymic Epithelial Cells-Brief Report.
In Arterioscler Thromb Vasc Biol on 1 July 2018 by Wilhelmson, A. S., Lantero Rodriguez, M., et al.
PubMed
Androgen deprivation therapy has been associated with increased cardiovascular risk in men. Experimental studies support that testosterone protects against atherosclerosis, but the target cell remains unclear. T cells are important modulators of atherosclerosis, and deficiency of testosterone or its receptor, the AR (androgen receptor), induces a prominent increase in thymus size. Here, we tested the hypothesis that atherosclerosis induced by testosterone deficiency in male mice is T-cell dependent. Further, given the important role of the thymic epithelium for T-cell homeostasis and development, we hypothesized that depletion of the AR in thymic epithelial cells will result in increased atherosclerosis.
-
-
-
Mus musculus (Mouse)
-
Genetics
-
Immunology and Microbiology
In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers.
In Nat Nanotechnol on 1 August 2017 by Smith, T. T., Stephan, S. B., et al.
PubMed
An emerging approach for treating cancer involves programming patient-derived T cells with genes encoding disease-specific chimeric antigen receptors (CARs), so that they can combat tumour cells once they are reinfused. Although trials of this therapy have produced impressive results, the in vitro methods they require to generate large numbers of tumour-specific T cells are too elaborate for widespread application to treat cancer patients. Here, we describe a method to quickly program circulating T cells with tumour-recognizing capabilities, thus avoiding these complications. Specifically, we demonstrate that DNA-carrying nanoparticles can efficiently introduce leukaemia-targeting CAR genes into T-cell nuclei, thereby bringing about long-term disease remission. These polymer nanoparticles are easy to manufacture in a stable form, which simplifies storage and reduces cost. Our technology may therefore provide a practical, broadly applicable treatment that can generate anti-tumour immunity 'on demand' for oncologists in a variety of settings.
-
-
Anti-CD3/Anti-CXCL10 Antibody Combination Therapy Induces a Persistent Remission of Type 1 Diabetes in Two Mouse Models.
In Diabetes on 1 December 2015 by Lasch, S., Müller, P., et al.
PubMed
Anti-CD3 therapy of type 1 diabetes results in a temporary halt of its pathogenesis but does not constitute a permanent cure. One problem is the reinfiltration of islets of Langerhans with regenerated, autoaggressive lymphocytes. We aimed at blocking such a reentry by neutralizing the key chemokine CXCL10. Combination therapy of diabetic RIP-LCMV and NOD mice with anti-CD3 and anti-CXCL10 antibodies caused a substantial remission of diabetes and was superior to monotherapy with anti-CD3 or anti-CXCL10 alone. The combination therapy prevented islet-specific T cells from reentering the islets of Langerhans and thereby blocked the autodestructive process. In addition, the local immune balance in the pancreas was shifted toward a regulatory phenotype. A sequential temporal inactivation of T cells and blockade of T-cell migration might constitute a novel therapy for patients with type 1 diabetes.