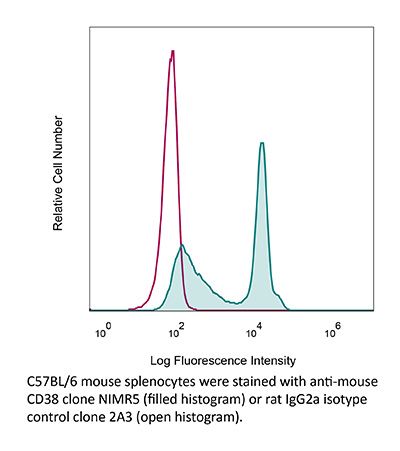
InVivoMAb anti-mouse CD38
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | BCL1 plasma membrane glycoproteins |
| Reported Applications |
in vivo CD38 stimulation in vitro CD38 stimulation in vitro B cell activation Immunofluorescence ELISA Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2754555 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CD38 stimulation
in vitro CD38 stimulation
Karakasheva, T. A., et al (2015). "CD38-Expressing Myeloid-Derived Suppressor Cells Promote Tumor Growth in a Murine Model of Esophageal Cancer" Cancer Res 75(19): 4074-4085.
PubMed
Myeloid-derived suppressor cells (MDSC) are an immunosuppressive population of immature myeloid cells found in advanced-stage cancer patients and mouse tumor models. Production of inducible nitric oxide synthase (iNOS) and arginase, as well as other suppressive mechanisms, allows MDSCs to suppress T-cell-mediated tumor clearance and foster tumor progression. Using an unbiased global gene expression approach in conditional p120-catenin knockout mice (L2-cre;p120ctn(f/f)), a model of oral-esophageal cancer, we have identified CD38 as playing a vital role in MDSC biology, previously unknown. CD38 belongs to the ADP-ribosyl cyclase family and possesses both ectoenzyme and receptor functions. It has been described to function in lymphoid and early myeloid cell differentiation, cell activation, and neutrophil chemotaxis. We find that CD38 expression in MDSCs is evident in other mouse tumor models of esophageal carcinogenesis, and CD38(high) MDSCs are more immature than MDSCs lacking CD38 expression, suggesting a potential role for CD38 in the maturation halt found in MDSC populations. CD38(high) MDSCs also possess a greater capacity to suppress activated T cells, and promote tumor growth to a greater degree than CD38(low) MDSCs, likely as a result of increased iNOS production. In addition, we have identified novel tumor-derived factors, specifically IL6, IGFBP3, and CXCL16, which induce CD38 expression by MDSCs ex vivo. Finally, we have detected an expansion of CD38(+) MDSCs in peripheral blood of advanced-stage cancer patients and validated targeting CD38 in vivo as a novel approach to cancer therapy.
in vitro CD38 stimulation
Flow Cytometry
ELISA
Lund, F. E., et al (2006). "CD38 induces apoptosis of a murine pro-B leukemic cell line by a tyrosine kinase-dependent but ADP-ribosyl cyclase- and NAD glycohydrolase-independent mechanism" Int Immunol 18(7): 1029-1042.
PubMed
Cross-linking of CD38 on hematopoietic cells induces activation, proliferation and differentiation of mature T and B cells and mediates apoptosis of myeloid and lymphoid progenitor cells. In addition to acting as a signaling receptor, CD38 is also an enzyme capable of producing several calcium-mobilizing metabolites, including cyclic adenosine diphosphate ribose (cADPR). It has been previously postulated that the calcium-mobilizing metabolites produced by CD38 may regulate its receptor-based activities. To test this hypothesis, we examined whether the enzyme activity of CD38 controls the apoptosis of an anti-CD38-stimulated leukemic B cell. We show that anti-CD38-induced apoptosis of Ba/F3 cells, a murine pro-B cell line, is not affected by blocking the calcium-mobilizing activity of cADPR or by inhibiting intracellular or extracellular calcium mobilization. In addition, we demonstrate that blocking CD38 enzyme activity with 2′-deoxy-2′-fluoro-nicotinamide arabinoside adenine dinucleotide has no effect on apoptosis and that Ba/F3 cells expressing catalytically inactive mutant forms of CD38 still undergo apoptosis upon CD38 cross-linking. Instead, we find that anti-CD38-induced apoptosis is dependent on tyrosine kinase and caspase activation, and that this process appears to be potentiated by the presence of membrane microdomains. Thus, the receptor-mediated functions of CD38 can be separated from its enzyme activity in a murine leukemic cell line, suggesting that CD38 plays multiple, but independent, biologic roles.
in vitro B cell stimulation
Chen, Q. and A. C. Ross (2005). "Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells" Proc Natl Acad Sci U S A 102(40): 14142-14149.
PubMed
Vitamin A and its active metabolite, all-trans retinoic acid (RA), regulate the antibody response in vivo, although the underlying mechanisms are not well understood. We have investigated the regulation by RA of B cell population dynamics and Ig gene expression in purified splenic mouse B cells stimulated through the B cell antigen receptor (BCR) and/or CD38, a BCR coreceptor. After ligation of the BCR and/or CD38, B cells became more heterogeneous in size. RA substantially restrained this change, concomitant with inhibition of cell proliferation. To examine B cell heterogeneity more closely, we categorized stimulated B cells by size (forward angle light scatter) and determined cell division dynamics, germ-line Ig heavy chain gene transcription and surface IgG1 (sIgG1) expression. Flow cytometric analysis of carboxyfluorescein diacetate succinimidyl ester-labeled B cells costained for sIgG1 showed that the more proliferative groups of B cells were smaller, whereas cells expressing more sIgG1 were larger. RA enriched the latter population, whereas cell division frequency in general and the number of smaller B cells that had undergone division cycles were reduced. Although RA significantly inhibited Ig germ-line transcript levels in the total B cell population, CD19(-)IgG1(+) B cells, which represent a more differentiated phenotype, were enriched. Furthermore, pax-5 mRNA was decreased and activation-induced cytidine deaminase mRNA was increased in RA-treated stimulated B cells. Thus, RA regulated factors known to be required for Ig class switch recombination and modulated the population dynamics of ligation-stimulated B cells, while promoting the progression of a fraction of B cells into differentiated sIgG-expressing cells.
in vitro B cell stimulation
Corcoran, L. M. and D. Metcalf (1999). "IL-5 and Rp105 signaling defects in B cells from commonly used 129 mouse substrains" J Immunol 163(11): 5836-5842.
PubMed
The use of 129 strain-derived embryonic stem cell lines for targeted gene mutation in mice has led directly to an expanded use of this inbred strain worldwide. It has been noted, however, that the 129 genetic background can make a significant contribution to the severity of a mutant phenotype. In this study, we reveal a specific defect in the IL-5 and Rp105 responses of B lymphocytes from two widely used 129 mouse substrains. The response to stimulation through surface IgM is also diminished, although to a lesser degree, in these mice. The lesion appears to reduce significantly the expression of the alpha-chain of the IL-5R, but may also influence events downstream of the IL-5R. This phenotype displays a codominant inheritance pattern, and is accompanied by a variable but significant depression of peritoneal B-1 cell numbers in 50% of the mice.
Immunofluorescence
Oliver, A. M., et al (1997). "Mouse CD38 is down-regulated on germinal center B cells and mature plasma cells" J Immunol 158(3): 1108-1115.
PubMed
Germinal center formation is the result of antigenic stimulation of B cells in a T cell-rich area. B cells cycle through the germinal centers, and a small percentage survive to become plasma cells or memory B cells. The transformation from a mature B cell into a germinal center B cell and finally into a terminally differentiated B cell is not well understood. Human CD38 is highly expressed on both germinal center B cells and plasma cells, and is useful in delineating these B cell subsets and in understanding the signaling events involved in the development of these B cells. To determine whether CD38 expression on activated germinal center B cells and postgerminal center B cells influences germinal center differentiation, we studied the expression of CD38 in the mouse. CD38 is expressed on follicular B cells in the Peyer’s patches but is down-regulated on germinal center B cells located within the Peyer’s patches. CD38dim/-B220+ germinal center B cells are also found in the spleens of immunized but not control mice, suggesting that Ag-stimulated germinal center formation is involved in the production of CD38dim/-B220+ B cells. Furthermore, mature plasma cells isolated from in vitro LPS cultures do not express CD38, but do contain high levels of cytoplasmic Ig. These results are in contrast to studies in humans in which CD38 is not found on follicular B cells but is highly expressed on germinal center B cells and plasma cells.
in vitro B cell stimulation
Kirkham, P. A., et al (1994). "Murine B-cell activation via CD38 and protein tyrosine phosphorylation" Immunology 83(4): 513-516.
PubMed
CD38 has been implicated in the regulation of both proliferation and rescue from apoptosis of B cells. The signalling events associated with CD38-mediated activation of murine B cells are, as yet, not well defined but it is clear that ligation of CD38 by a mitogenic antibody, NIMR-5, induces a calcium influx in resting B cells. Interestingly, however, cross-linking of CD38 does not mobilize intracellular stores of calcium. We now provide a rationale for these findings by demonstrating that CD38 is not coupled to the generation of inositol phosphates in resting B cells. We do, however, show that CD38 ligation stimulates one, or more, protein tyrosine kinase activities which may play a central role in the transduction of CD38-mediated signals leading to B-cell activation.
Product Citations
-
-
Cancer Research
Tumor suppressors in Sox2-mediated lung cancers promote distinct cell-intrinsic and immunologic remodeling.
In JCI Insight on 23 June 2025 by Sengottuvel, N., Whately, K. M., et al.
PubMed
Non-small cell lung cancer (NSCLC) largely consists of lung squamous carcinoma (LUSC) and lung adenocarcinoma (LUAD). Alterations in the tumor protein p53 (TP53) and phosphatase and tensin homolog (PTEN) tumor suppressors are common in both subtypes, but their relationship with SOX2 is poorly understood. We deleted Trp53 or Pten in a C57BL/6 Sox2hi Nkx2-1-/- Lkb1-/- (SNL) genetic background and generated a highly metastatic LUSC cell line (LN2A; derived from a Sox2hi mouse model, followed by Trp53, Pten, and cyclin dependent kinase inhibitor 2A [Cdkn2a] deletion). Histologic and single-cell RNA-Seq analyses corroborated that SNL mice developed mixed tumors with both LUAD and LUSC histopathology while SNL-Trp53 and SNL-Pten mice developed LUAD and LN2A tumors that retained LUSC morphology. Compared with SNL mice, additional loss of Trp53 or Pten resulted in significantly reduced survival, increased tumor burden, and altered tumor mucin composition. We identified a subcluster of CD38+ tumor-associated inflammatory monocytes in the LN2A model that was significantly enriched for activation of the classical and alternative complement pathways. Complement factor B (CFB) is associated with poor survival in patients with LUSC, and we observed the LN2A model had significantly improved survival on a Cfb-/- background. Our findings demonstrate a cooperative role of Trp53 and Pten tumor suppressors in Sox2-mediated NSCLC tumor progression, mucin production, and remodeling of the immune tumor microenvironment.
-
-
-
Cancer Research
-
Immunology and Microbiology
Neoantigen Dendritic Cell Vaccination Combined with Anti-CD38 and CpG Elicits Anti-Tumor Immunity against the Immune Checkpoint Therapy-Resistant Murine Lung Cancer Cell Line LLC1.
In Cancers (Basel) on 2 November 2021 by Sun, C., Nagaoka, K., et al.
PubMed
An important factor associated with primary resistance to immune-checkpoint therapies (ICT) is a "cold" tumor microenvironment (TME), characterized by the absence of T cell infiltration and a non-inflammatory milieu. Whole-exome and RNA sequencing to predict neoantigen expression was performed on the LLC1 cell line which forms "cold" tumors in mice. Dendritic cell (DC)-based vaccination strategies were developed using candidate neoantigen long peptides (LPs). A total of 2536 missense mutations were identified in LLC1 and of 132 candidate neoantigen short peptides, 25 were found to induce CD8+ T cell responses. However, they failed to inhibit LLC1 growth when incorporated into a cancer vaccine. In contrast, DCs pulsed with LPs induced CD4+ and CD8+ T cell responses and one of them, designated L82, delayed LLC1 growth in vivo. By RNA-Seq, CD38 was highly expressed by LLC1 tumor cells and, therefore, anti-CD38 antibody treatment was combined with L82-pulsed DC vaccination. This combination effectively suppressed tumor growth via a mechanism relying on decreased regulatory T cells in the tumor. This study demonstrated that an appropriate vaccination strategy combining neoantigen peptide-pulsed DC with anti-CD38 antibody can render an ICT-resistant "cold" tumor susceptible to immune rejection via a mechanism involving neutralization of regulatory T cells.
-