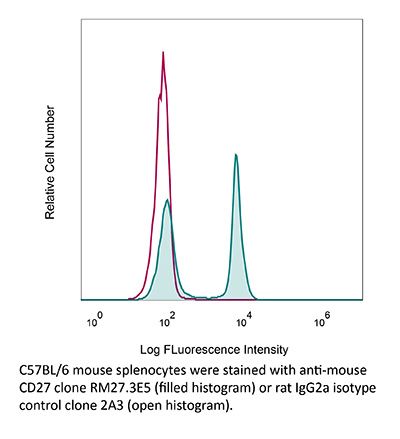
InVivoMAb anti-mouse CD27
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse CD27-human IgG1 Fc fusion protein |
| Reported Applications |
in vivo CD27 stimulation in vitro CD27 stimulation Immunoprecipitation Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2894767 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo CD27 stimulation
in vitro CD27 stimulation
Ahrends, T., et al (2017). "CD4(+) T Cell Help Confers a Cytotoxic T Cell Effector Program Including Coinhibitory Receptor Downregulation and Increased Tissue Invasiveness" Immunity 47(5): 848-861 e845.
PubMed
CD4(+) T cells optimize the cytotoxic T cell (CTL) response in magnitude and quality, by unknown molecular mechanisms. We here present the transcriptomic changes in CTLs resulting from CD4(+) T cell help after anti-cancer vaccination or virus infection. The gene expression signatures revealed that CD4(+) T cell help during priming optimized CTLs in expression of cytotoxic effector molecules and many other functions that ensured efficacy of CTLs throughout their life cycle. Key features included downregulation of PD-1 and other coinhibitory receptors that impede CTL activity, and increased motility and migration capacities. “Helped” CTLs acquired chemokine receptors that helped them reach their tumor target tissue and metalloprotease activity that enabled them to invade into tumor tissue. A very large part of the “help” program was instilled in CD8(+) T cells via CD27 costimulation. The help program thus enhances specific CTL effector functions in response to vaccination or a virus infection.
in vivo CD27 stimulation
Ahrends, T., et al (2016). "CD27 Agonism Plus PD-1 Blockade Recapitulates CD4+ T-cell Help in Therapeutic Anticancer Vaccination" Cancer Res 76(10): 2921-2931.
PubMed
While showing promise, vaccination strategies to treat cancer require further optimization. Likely barriers to efficacy involve cancer-associated immunosuppression and peripheral tolerance, which limit the generation of effective vaccine-specific cytotoxic T lymphocytes (CTL). Because CD4(+) T cells improve CTL responsiveness, next-generation vaccines include helper epitopes. Here, we demonstrate in mice how CD4(+) T-cell help optimizes the CTL response to a clinically relevant DNA vaccine engineered to combat human papillomavirus-expressing tumors. Inclusion of tumor-unrelated helper epitopes greatly increased CTL priming, effector, and memory T-cell programming. CD4(+) T-cell help optimized the CTL response in all these aspects via CD27/CD70 costimulation. Notably, administration of an agonistic CD27 antibody could largely replace helper epitopes in promoting primary and memory CTL responses, acting directly on CD8(+) T cells. CD27 agonism improved efficacy of the vaccine without helper epitopes, more so than combined PD-1 and CTLA-4 blockade. Combining CD27 agonism with CTLA-4 blockade improved vaccine-induced CTL priming and tumor infiltration, but only combination with PD-1 blockade was effective at eradicating tumors, thereby fully recapitulating the effect of CD4(+) T-cell help on vaccine efficacy. PD-1 blockade alone did not affect CTL priming or tumor infiltration, so these results implied that it cooperated with CD4(+) T-cell help by alleviating immune suppression against CTL in the tumor. Helper epitope inclusion or CD27 agonism did not stimulate regulatory T cells, and vaccine efficacy was also improved by CD27 agonism in the presence of CD4(+) T-cell help. Our findings provide a preclinical rationale to apply CD27 agonist antibodies, either alone or combined with PD-1 blockade, to improve the therapeutic efficacy of cancer vaccines and immunotherapy generally. Cancer Res; 76(10); 2921-31. (c)2016 AACR.
in vivo CD27 stimulation
Ngiow, S. F., et al (2016). "Agonistic CD40 mAb-Driven IL12 Reverses Resistance to Anti-PD1 in a T-cell-Rich Tumor" Cancer Res 76(21): 6266-6277.
PubMed
The durability and efficacy of anti-human PD1 monoclonal antibodies (PD1 mAb) vary across different malignancies. Although an absence of tumor-infiltrating cytotoxic T lymphocytes has been identified as a cause for resistance to PD1 mAb, the presence of intratumor exhausted PD1(hi) T cells also contributes to insensitivity to this immune checkpoint therapy. In this study, we used mouse tumor models of PD1 mAb resistance that harbored PD1(hi) T cells and flow cytometry analysis of tumor-infiltrating leukocytes immediately post-therapy as a screening platform to identify agents that could resensitize T cells to PD1 blockade. We showed that an agonistic anti-CD40 mAb converted PD1(hi) T cells into PD1(lo) T cells, reversing phenotypic T-cell exhaustion and allowing the anti-PD1 refractory tumors to respond to anti-PD1 therapy. PD1 downmodulation by anti-CD40 mAb relied upon IL12 but not IL23, CD80/CD86/CD28, or CD70/CD27. Consistent with a role for regulatory T cells (Treg) in promoting T-cell exhaustion, we also showed that intratumor Treg presented with a less activated and attenuated suppressive phenotype, marked by reductions in CTLA4 and PD1. Similar to anti-CD40 mAb, anti-CTLA4 mAb also lowered intratumor T-cell PD1 expression. Our study provides a proof-of-principle framework to systematically identify immune conditioning agents able to convert PD1(hi) T cells to PD1(lo) T cells, with clinical implications in the management of anti-PD1 refractory patients. Cancer Res; 76(21); 6266-77. (c)2016 AACR.
in vivo CD27 stimulation
in vitro CD27 stimulation
Flow Cytometry
Immunoprecipitation
Roberts, D. J., et al (2010). "Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells" J Immunother 33(8): 769-779.
PubMed
The immune response to the tumor can be enhanced by targeting costimulatory molecules on T cells. As the CD70-CD27 costimulatory axis plays an important role in the activation, survival, and differentiation of lymphocytes, we have examined the efficacy of agonistic anti-CD27 antibodies as monotherapies for established melanoma in a murine model. We show that this approach leads to a substantial reduction in the outgrowth of both experimental lung metastases and subcutaneous tumors. Anti-CD27 treatment supports the maintenance of tumor-specific CD8(+) T cells within the tumor, reduces the frequency of FoxP3-expressing CD4(+) T cells within tumors, and potentiates the ability of NK1.1(+) and CD8(+) tumor infiltrating cells to secrete IFNgamma upon coculture with tumor cells. The enhanced effector function correlated with lower levels of PD-1 expression on CD8(+) T cells from anti-CD27-treated mice. Despite the modulating effect of anti-CD27 on multiple cell types, only CD8(+) T cells were absolutely required for tumor control. The CD4(+) T cells were dispensable, whereas NK1.1(+) cells were needed during early stages of tumor growth but not for the effectiveness of anti-CD27. Thus, CD27-mediated costimulation provides a potent boost to multiple aspects of the endogenous responses to tumor, and may be exploited to enhance tumor immunity.
in vivo CD27 stimulation
Sakanishi, T. and H. Yagita (2010). "Anti-tumor effects of depleting and non-depleting anti-CD27 monoclonal antibodies in immune-competent mice" Biochem Biophys Res Commun 393(4): 829-835.
PubMed
CD27 plays an important role in T-cell co-stimulation and is also expressed on lymphomas. In the present study, we generated novel depleting and non-depleting monoclonal antibodies (mAbs) against mouse CD27 and characterized their co-stimulatory activity in vitro and anti-tumor effects in immune-competent mice bearing syngeneic T-cell lymphoma (EG7) expressing or lacking CD27. A profound anti-tumor effect was observed with a non-depleting mAb (RM27-3E5), but not with a depleting mAb (RM27-3C1), against either EG7/CD27(+) or EG7/CD27(-) tumors, which was associated with the induction of EG7-specific cytotoxic T lymphocytes (CTLs). Consistently, the anti-tumor effect of RM27-3E5 was abolished in T cell-deficient nude mice. These results indicate that a non-depleting agonistic mAb against CD27 is promising for cancer therapy by co-stimulating tumor-specific CTL induction.
in vivo CD27 stimulation
in vitro CD27 stimulation
Sumi, T., et al (2008). "CD27 and CD70 do not play a critical role in the development of experimental allergic conjunctivitis in mice" Immunol Lett 119(1-2): 91-96.
PubMed
CD27, which belongs to the TNF receptor family, is a costimulatory molecule that participates in T-cell activation. Unlike costimulatory molecules such as OX40 and 4-1BB, little is known about the role CD27 plays a role in the development of experimental diseases. We asked whether CD27 and its ligand CD70 participate in the development of experimental allergic conjunctivitis (EC) in BALB/c mice, which is generated by immunization with ragweed (RW) in alum and challenged 10 days later with RW in eye drops. The roles of CD27 and CD70 were tested by intraperitoneally injecting the mice with anti-CD27, anti-CD70 or a control Ab during the induction or effector phase. Twenty-four hours after challenge, the conjunctivas, blood and spleens were harvested for histological analysis, measuring Ig levels and cytokine analysis, respectively. Regardless of when the mice were treated, anti-CD27 or anti-CD70 Ab treatment did not significantly affect the severity of EC as evaluated by conjunctival eosinophil numbers. However, anti-CD27 or anti-CD70 Ab treatment during the induction phase did significantly modulate systemic humoral and cellular immune responses. In vitro treatment of RW-primed splenocytes with anti-CD27 or anti-CD70 Ab did not affect the EC-inducing capability of the splenocytes. Taken together, CD27 and CD70 do not play a critical role in the development of EC.
Product Citations
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Small molecules from antibody pharmacophores (SMAbPs) as a hit identification workflow for immune checkpoints.
In Sci Adv on 18 October 2024 by Abdel-Rahman, S. A., Gabr, M. T., et al.
PubMed
Small-molecule modulators of immune checkpoints are poised to revolutionize cancer immunotherapy. However, efficient strategies for hit identification are lacking. We introduce small molecules from antibody pharmacophores (SMAbPs), a workflow leveraging cocrystal structures of checkpoints with antibodies to create pharmacophore maps for virtual screening. Applying SMAbPs to five immune checkpoints yielded hits with submicromolar potency in both cell-free and cellular assays. Notably, SMAbPs identified the most potent T cell immunoglobulin and mucin-domain containing-3 and V-domain immunoglobulin suppressor of T cell activation (VISTA) inhibitors reported to date and first-in-class modulators of B and T lymphocyte attenuator, 4-IBB, and CD27. Targeting inhibitory and costimulatory checkpoints with hits identified through SMAbPs demonstrated remarkable in vivo antitumor activity, exemplified by MG-V-53 (VISTA inhibitor) and MG-C-30 (CD27 agonist), which significantly reduced tumor volumes in MC38 and EG7-OVA mouse models, respectively.
-
-
-
Flow cytometry/Cell sorting
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Chronic CD27-CD70 costimulation promotes type 1-specific polarization of effector Tregs.
In Front Immunol on 31 March 2023 by Bowakim-Anta, N., Acolty, V., et al.
PubMed
Most T lymphocytes, including regulatory T cells, express the CD27 costimulatory receptor in steady state conditions. There is evidence that CD27 engagement on conventional T lymphocytes favors the development of Th1 and cytotoxic responses in mice and humans, but the impact on the regulatory lineage is unknown.
-