InVivoMAb anti-mouse CD20
Product Description
*Please note that the AISB12 clone is not suitable for in vivo B cell depletion. This antibody has little to no B cell depleting activity.
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
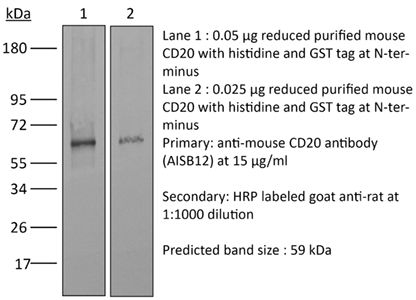
| Immunogen | Full length mouse CD20 protein |
| Reported Applications |
Flow cytometry Western blot Not recommended for in vivo B cell depletion |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2715460 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Flow Cytometry
Mishima, Y., et al (2010). "Decreased production of interleukin-10 and transforming growth factor-beta in Toll-like receptor-activated intestinal B cells in SAMP1/Yit mice" Immunology 131(4): 473-487.
PubMed
A unique subset of B cells expressing interleukin-10 (IL-10) and transforming growth factor-beta (TGF-beta) plays an essential role in preventing inflammation and autoimmunity. We investigated the presence of this cell subset in intestines and its role in the pathogenesis of ileitis using SAMP1/Yit and age-matched control AKR/J mice. Mononuclear cells were isolated from mesenteric lymph nodes (MLNs) and the expressions of B220, CD1d, CD5, Toll-like receptor 4 (TLR4) and TLR9 in isolated cells were analysed. Purified B cells were stimulated with lipopolysaccharide (LPS) or CpG-DNA, then IL-10 and TGF-beta(1) expressions were examined by enzyme immunoassay and flow cytometry. Production of IL-1beta by TLR-mediated macrophages co-cultured with or without purified MLN B cells from SAMP1/Yit and AKR/J mice was evaluated. In addition, interferon-gamma (IFN-gamma) production in intestinal T cells co-cultured with MLN B cells were also assessed in SAMP1/Yit and AKR/J strains. The production levels of IL-10 and TGF-beta(1) stimulated by LPS and CpG-DNA were significantly lower in B cells separated from MLNs from the SAMP1/Yit strain. B cells expressing IL-10 and TGF-beta(1) were mainly located in a population characterized by the cell surface marker CD1d(+) . Interleukin-1beta production by TLR-activated macrophages co-cultured with MLN B cells from SAMP1/Yit mice was significantly higher than that of those from AKR/J mice. Interestingly, IFN-gamma production by T cells was noted only when they were co-cultured with SAMP1/Yit but not the AKR/J B cells. These results are the first to show that disorders of regulatory B-cell function under innate immune activation may cause disease pathogenesis in a murine model of Crohn’s disease.
Product Citations
-
-
Genetics
-
Immunology and Microbiology
Immune profiling of adeno-associated virus response identifies B cell-specific targets that enable vector re-administration in mice.
In Gene Ther on 1 May 2023 by Chen, M., Kim, B., et al.
PubMed
Adeno-associated virus (AAV) vector-based gene therapies can be applied to a wide range of diseases. AAV expression can last for months to years, but vector re-administration may be necessary to achieve life-long treatment. Unfortunately, immune responses against these vectors are potentiated after the first administration, preventing the clinical use of repeated administration of AAVs. Reducing the immune response against AAVs while minimizing broad immunosuppression would improve gene delivery efficiency and long-term safety. In this study, we quantified the contributions of multiple immune system components of the anti-AAV response in mice. We identified B-cell-mediated immunity as a critical component preventing vector re-administration. Additionally, we found that IgG depletion alone was insufficient to enable re-administration, suggesting IgM antibodies play an important role in the immune response against AAV. Further, we found that AAV-mediated transduction is improved in µMT mice that lack functional IgM heavy chains and cannot form mature B-cells relative to wild-type mice. Combined, our results suggest that B-cells, including non-class switched B-cells, are a potential target for therapeutics enabling AAV re-administration. Our results also suggest that the µMT mice are a potentially useful experimental model for gene delivery studies since they allow repeated dosing for more efficient gene delivery from AAVs.
-
-
Targeted immunosuppression enhances repeated gene delivery
In Research Square on 1 March 2022 by Chen, M., Kim, B., et al.
-
-
Immunology and Microbiology
Regulatory T Cell-Derived TGF-β1 Controls Multiple Checkpoints Governing Allergy and Autoimmunity.
In Immunity on 15 December 2020 by Turner, J. A., Stephen-Victor, E., et al.
PubMed
The mechanisms by which regulatory T (Treg) cells differentially control allergic and autoimmune responses remain unclear. We show that Treg cells in food allergy (FA) had decreased expression of transforming growth factor beta 1 (TGF-β1) because of interleukin-4 (IL-4)- and signal transducer and activator of transciription-6 (STAT6)-dependent inhibition of Tgfb1 transcription. These changes were modeled by Treg cell-specific Tgfb1 monoallelic inactivation, which induced allergic dysregulation by impairing microbiota-dependent retinoic acid receptor-related orphan receptor gamma t (ROR-γt)+ Treg cell differentiation. This dysregulation was rescued by treatment with Clostridiales species, which upregulated Tgfb1 expression in Treg cells. Biallelic deficiency precipitated fatal autoimmunity with intense autoantibody production and dysregulated T follicular helper and B cell responses. These results identify a privileged role of Treg cell-derived TGF-β1 in regulating allergy and autoimmunity at distinct checkpoints in a Tgfb1 gene dose- and microbiota-dependent manner.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
Trispecific natural killer cell nanoengagers for targeted chemoimmunotherapy.
In Sci Adv on 1 July 2020 by Au, K. M., Park, S. I., et al.
PubMed
Activation of the innate immune system and natural killer (NK) cells has been a key effort in cancer immunotherapy research. Here, we report a nanoparticle-based trispecific NK cell engager (nano-TriNKE) platform that can target epidermal growth factor receptor (EGFR)-overexpressing tumors and promote the recruitment and activation of NK cells to eradicate these cancer cells. Moreover, the nanoengagers can deliver cytotoxic chemotherapeutics to further improve their therapeutic efficacy. We have demonstrated that effective NK cell activation can be achieved by the spatiotemporal coactivation of CD16 and 4-1BB stimulatory molecules on NK cells with nanoengagers, and the nanoengagers are more effective than free antibodies. We also show that biological targeting, either through radiotherapy or EGFR, is critical to the therapeutic effects of nanoengagers. Last, EGFR-targeted nanoengagers can augment both NK-activating agents and chemotherapy (epirubicin) as highly effective anticancer agents, providing robust chemoimmunotherapy.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity.
In Cell on 19 March 2020 by Lu, Y., Zhao, Q., et al.
PubMed
Understanding molecular mechanisms that dictate B cell diversity is important for targeting B cells as anti-cancer treatment. Through the single-cell dissection of B cell heterogeneity in longitudinal samples of patients with breast cancer before and after neoadjuvant chemotherapy, we revealed that an ICOSL+ B cell subset emerges after chemotherapy. Using three immunocompetent mouse models, we recapitulated the subset switch of human tumor-infiltrating B cells during chemotherapy. By employing B-cell-specific deletion mice, we showed that ICOSL in B cells boosts anti-tumor immunity by enhancing the effector to regulatory T cell ratio. The signature of ICOSL+ B cells is imprinted by complement-CR2 signaling, which is triggered by immunogenic cell death. Moreover, we identified that CD55, a complement inhibitory protein, determines the opposite roles of B cells in chemotherapy. Collectively, we demonstrated a critical role of the B cell subset switch in chemotherapy response, which has implications in designing novel anti-cancer therapies. VIDEO ABSTRACT.
-
-
-
In vivo experiments
-
In vivo experiments
-
Mus musculus (Mouse)
-
Biochemistry and Molecular biology
-
Endocrinology and Physiology
-
Immunology and Microbiology
Gut Microbiota-Stimulated Innate Lymphoid Cells Support β-Defensin 14 Expression in Pancreatic Endocrine Cells, Preventing Autoimmune Diabetes.
In Cell Metab on 2 October 2018 by Miani, M., Le Naour, J., et al.
PubMed
The gut microbiota is essential for the normal function of the gut immune system, and microbiota alterations are associated with autoimmune disorders. However, how the gut microbiota prevents autoimmunity in distant organs remains poorly defined. Here we reveal that gut microbiota conditioned innate lymphoid cells (ILCs) induce the expression of mouse β-defensin 14 (mBD14) by pancreatic endocrine cells, preventing autoimmune diabetes in the non-obese diabetic (NOD) mice. MBD14 stimulates, via Toll-like receptor 2, interleukin-4 (IL-4)-secreting B cells that induce regulatory macrophages, which in turn induce protective regulatory T cells. The gut microbiota-derived molecules, aryl hydrocarbon receptor (AHR) ligands and butyrate, promote IL-22 secretion by pancreatic ILCs, which induce expression of mBD14 by endocrine cells. Dysbiotic microbiota and low-affinity AHR allele explain the defective pancreatic expression of mBD14 observed in NOD mice. Our study reveals a yet unidentified crosstalk between ILCs and endocrine cells in the pancreas that is essential for the prevention of autoimmune diabetes development.
-