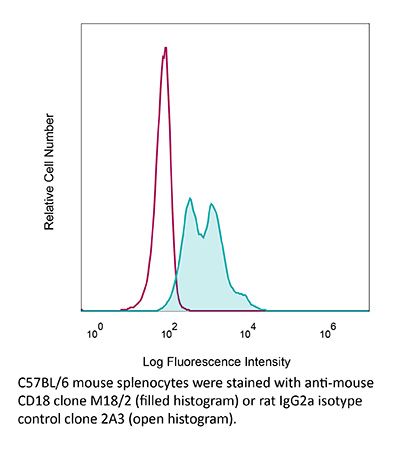
InVivoMAb anti-mouse CD18
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | C57BL/10 splenocytes |
| Reported Applications | in vivo LFA-1 neutralization |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107607 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo LFA-1 neutralization
He, W., et al (2018). "Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues" Immunity 49(6): 1175-1190.e1177.
PubMed
The number of leukocytes present in circulation varies throughout the day, reflecting bone marrow output and emigration from blood into tissues. Using an organism-wide circadian screening approach, we detected oscillations in pro-migratory factors that were distinct for specific vascular beds and individual leukocyte subsets. This rhythmic molecular signature governed time-of-day-dependent homing behavior of leukocyte subsets to specific organs. Ablation of BMAL1, a transcription factor central to circadian clock function, in endothelial cells or leukocyte subsets demonstrated that rhythmic recruitment is dependent on both microenvironmental and cell-autonomous oscillations. These oscillatory patterns defined leukocyte trafficking in both homeostasis and inflammation and determined detectable tumor burden in blood cancer models. Rhythms in the expression of pro-migratory factors and migration capacities were preserved in human primary leukocytes. The definition of spatial and temporal expression profiles of pro-migratory factors guiding leukocyte migration patterns to organs provides a resource for the further study of the impact of circadian rhythms in immunity.
in vivo LFA-1 neutralization
Zumwalde, N. A., et al (2013). "ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation" J Immunol 191(7): 3681-3693.
PubMed
A hallmark of T cell activation in vitro and in vivo is the clustering of T cells with each other via interaction of the LFA-1 integrin with ICAM-1. The functional significance of these homotypic aggregates in regulating T cell function remains unknown. We used an APC-free in vitro activation system to demonstrate that stimulation of purified naive CD8 T cells results in enhanced expression of ICAM-1 on T cells that is sustained by the inflammatory cytokine IL-12 and associated with robust T cell aggregates. ICAM-1-deficient CD8 T cells proliferate normally but demonstrate a striking failure to aggregate. Interestingly, loss of ICAM-1 expression results in elevated levels of IFN-gamma and granzyme B, as well as enhanced cytotoxicity. Similar results were obtained when anti-LFA-1 Ab was used to block the clustering of wild-type T cells. ICAM-1 ligation is not required for IFN-gamma regulation, as clustering of ICAM-1-deficient CD8 T cells with wild-type T cells reduces IFN-gamma expression. Analysis using a fluorescent reporter that monitors TCR signal strength indicates that T cell clustering limits T cell exposure to Ag during activation. Furthermore, T cell clustering promotes the upregulation of the CTLA-4 inhibitory receptor and the downregulation of eomesodermin, which controls effector molecule expression. Activation of ICAM-1-deficient CD8 T cells in vivo results in an enhanced percentage of KLRG-1(+) T cells indicative of short-lived effectors. These results suggest that T cell clustering represents a mechanism that allows continued proliferation but regulates T cell effector function and differentiation.
Product Citations
-
-
Cardiovascular biology
Acute kidney injury triggers hypoxemia by lung intravascular neutrophil retention that reduces capillary blood flow.
In J Clin Invest on 15 May 2025 by Komaru, Y., Ning, L., et al.
PubMed
Sterile acute kidney injury (AKI) is common in the clinic and frequently associated with unexplained hypoxemia that does not improve with dialysis. AKI induces remote lung inflammation with neutrophil recruitment in mice and humans, but which cellular cues establish neutrophilic inflammation and how it contributes to hypoxemia is not known. Here we report that AKI induced rapid intravascular neutrophil retention in lung alveolar capillaries without extravasation into tissue or alveoli, causing hypoxemia by reducing lung capillary blood flow in the absence of substantial lung interstitial or alveolar edema. In contrast to direct ischemic lung injury, lung neutrophil recruitment during remote lung inflammation did not require cues from intravascular nonclassical monocytes or tissue-resident alveolar macrophages. Instead, lung neutrophil retention depended on the neutrophil chemoattractant CXCL2 released by activated classical monocytes. Comparative single-cell RNA-Seq analysis of direct and remote lung inflammation revealed that alveolar macrophages were highly activated and produced CXCL2 only in direct lung inflammation. Establishing a CXCL2 gradient into the alveolus by intratracheal CXCL2 administration during AKI-induced remote lung inflammation enabled neutrophils to extravasate. We thus discovered important differences in lung neutrophil recruitment in direct versus remote lung inflammation and identified lung capillary neutrophil retention that negatively affected oxygenation by causing a ventilation-perfusion mismatch as a driver of AKI-induced hypoxemia.
-
-
-
In vivo experiments
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues.
In Immunity on 18 December 2018 by He, W., Holtkamp, S., et al.
PubMed
The number of leukocytes present in circulation varies throughout the day, reflecting bone marrow output and emigration from blood into tissues. Using an organism-wide circadian screening approach, we detected oscillations in pro-migratory factors that were distinct for specific vascular beds and individual leukocyte subsets. This rhythmic molecular signature governed time-of-day-dependent homing behavior of leukocyte subsets to specific organs. Ablation of BMAL1, a transcription factor central to circadian clock function, in endothelial cells or leukocyte subsets demonstrated that rhythmic recruitment is dependent on both microenvironmental and cell-autonomous oscillations. These oscillatory patterns defined leukocyte trafficking in both homeostasis and inflammation and determined detectable tumor burden in blood cancer models. Rhythms in the expression of pro-migratory factors and migration capacities were preserved in human primary leukocytes. The definition of spatial and temporal expression profiles of pro-migratory factors guiding leukocyte migration patterns to organs provides a resource for the further study of the impact of circadian rhythms in immunity.
-
-
-
Immunology and Microbiology
ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation.
In J Immunol on 1 October 2013 by Zumwalde, N. A., Domae, E., et al.
PubMed
A hallmark of T cell activation in vitro and in vivo is the clustering of T cells with each other via interaction of the LFA-1 integrin with ICAM-1. The functional significance of these homotypic aggregates in regulating T cell function remains unknown. We used an APC-free in vitro activation system to demonstrate that stimulation of purified naive CD8 T cells results in enhanced expression of ICAM-1 on T cells that is sustained by the inflammatory cytokine IL-12 and associated with robust T cell aggregates. ICAM-1-deficient CD8 T cells proliferate normally but demonstrate a striking failure to aggregate. Interestingly, loss of ICAM-1 expression results in elevated levels of IFN-γ and granzyme B, as well as enhanced cytotoxicity. Similar results were obtained when anti-LFA-1 Ab was used to block the clustering of wild-type T cells. ICAM-1 ligation is not required for IFN-γ regulation, as clustering of ICAM-1-deficient CD8 T cells with wild-type T cells reduces IFN-γ expression. Analysis using a fluorescent reporter that monitors TCR signal strength indicates that T cell clustering limits T cell exposure to Ag during activation. Furthermore, T cell clustering promotes the upregulation of the CTLA-4 inhibitory receptor and the downregulation of eomesodermin, which controls effector molecule expression. Activation of ICAM-1-deficient CD8 T cells in vivo results in an enhanced percentage of KLRG-1(+) T cells indicative of short-lived effectors. These results suggest that T cell clustering represents a mechanism that allows continued proliferation but regulates T cell effector function and differentiation.
-