InVivoMAb anti-mouse CD103
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse intestinal epithelial cells |
| Reported Applications |
in vivo CD103 neutralization Immunofluorescence Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107570 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
In vivo CD103 neutralization
Liikanen, I., et al (2021). "Hypoxia-inducible factor activity promotes antitumor effector function and tissue residency by CD8+ T cells" J Clin Invest 131(7).
PubMed
Adoptive T cell therapies (ACTs) hold great promise in cancer treatment, but low overall response rates in patients with solid tumors underscore remaining challenges in realizing the potential of this cellular immunotherapy approach. Promoting CD8+ T cell adaptation to tissue residency represents an underutilized but promising strategy to improve tumor-infiltrating lymphocyte (TIL) function. Here, we report that deletion of the HIF negative regulator von Hippel-Lindau (VHL) in CD8+ T cells induced HIF-1α/HIF-2α-dependent differentiation of tissue-resident memory-like (Trm-like) TILs in mouse models of malignancy. VHL-deficient TILs accumulated in tumors and exhibited a core Trm signature despite an exhaustion-associated phenotype, which led to retained polyfunctionality and response to αPD-1 immunotherapy, resulting in tumor eradication and protective tissue-resident memory. VHL deficiency similarly facilitated enhanced accumulation of chimeric antigen receptor (CAR) T cells with a Trm-like phenotype in tumors. Thus, HIF activity in CD8+ TILs promotes accumulation and antitumor activity, providing a new strategy to enhance the efficacy of ACTs.
In vivo CD103 neutralization
Homet Moreno, B., et al (2016). "Response to Programmed Cell Death-1 Blockade in a Murine Melanoma Syngeneic Model Requires Costimulation, CD4, and CD8 T Cells" Cancer Immunol Res 4(10): 845-857.
PubMed
The programmed cell death protein 1 (PD-1) limits effector T-cell functions in peripheral tissues, and its inhibition leads to clinical benefit in different cancers. To better understand how PD-1 blockade therapy modulates the tumor-host interactions, we evaluated three syngeneic murine tumor models, the BRAF(V600E)-driven YUMM1.1 and YUMM2.1 melanomas, and the carcinogen-induced murine colon adenocarcinoma MC38. The YUMM cell lines were established from mice with melanocyte-specific BRAF(V600E) mutation and PTEN loss (BRAF(V600E)/PTEN(-/-)). Anti-PD-1 or anti-PD-L1 therapy engendered strong antitumor activity against MC38 and YUMM2.1, but not YUMM1.1. PD-L1 expression did not differ between the three models at baseline or upon interferon stimulation. Whereas mutational load was high in MC38, it was lower in both YUMM models. In YUMM2.1, the antitumor activity of PD-1 blockade had a critical requirement for both CD4 and CD8 T cells, as well as CD28 and CD80/86 costimulation, with an increase in CD11c(+)CD11b(+)MHC-II(high) dendritic cells and tumor-associated macrophages in the tumors after PD-1 blockade. Compared with YUMM1.1, YUMM2.1 exhibited a more inflammatory profile by RNA sequencing analysis, with an increase in expression of chemokine-trafficking genes that are related to immune cell recruitment and T-cell priming. In conclusion, response to PD-1 blockade therapy in tumor models requires CD4 and CD8 T cells and costimulation that is mediated by dendritic cells and macrophages.
Immunofluorescence
Flow Cytometry
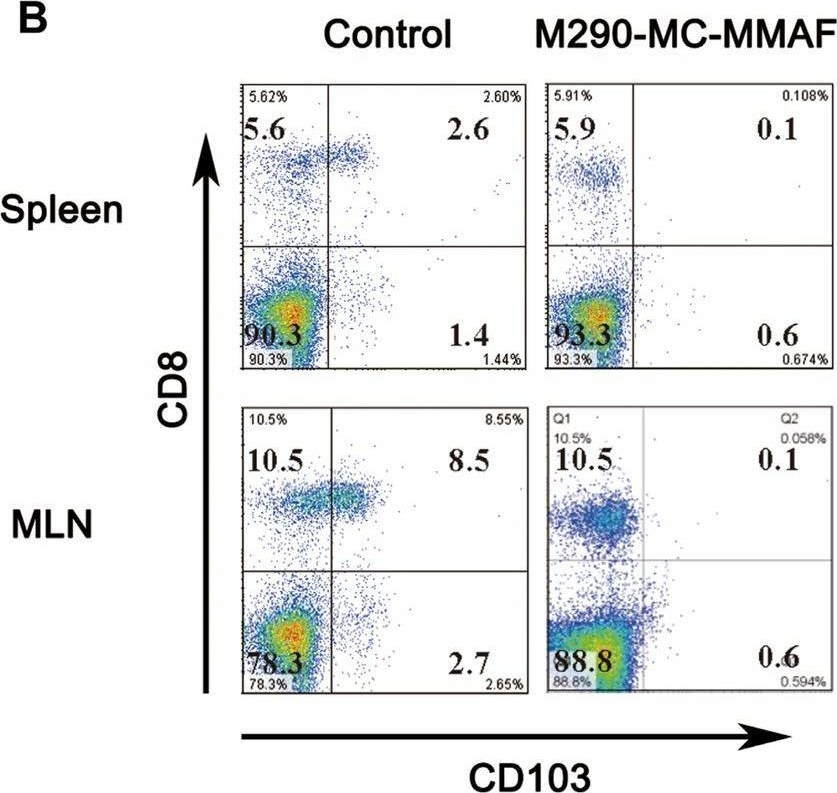
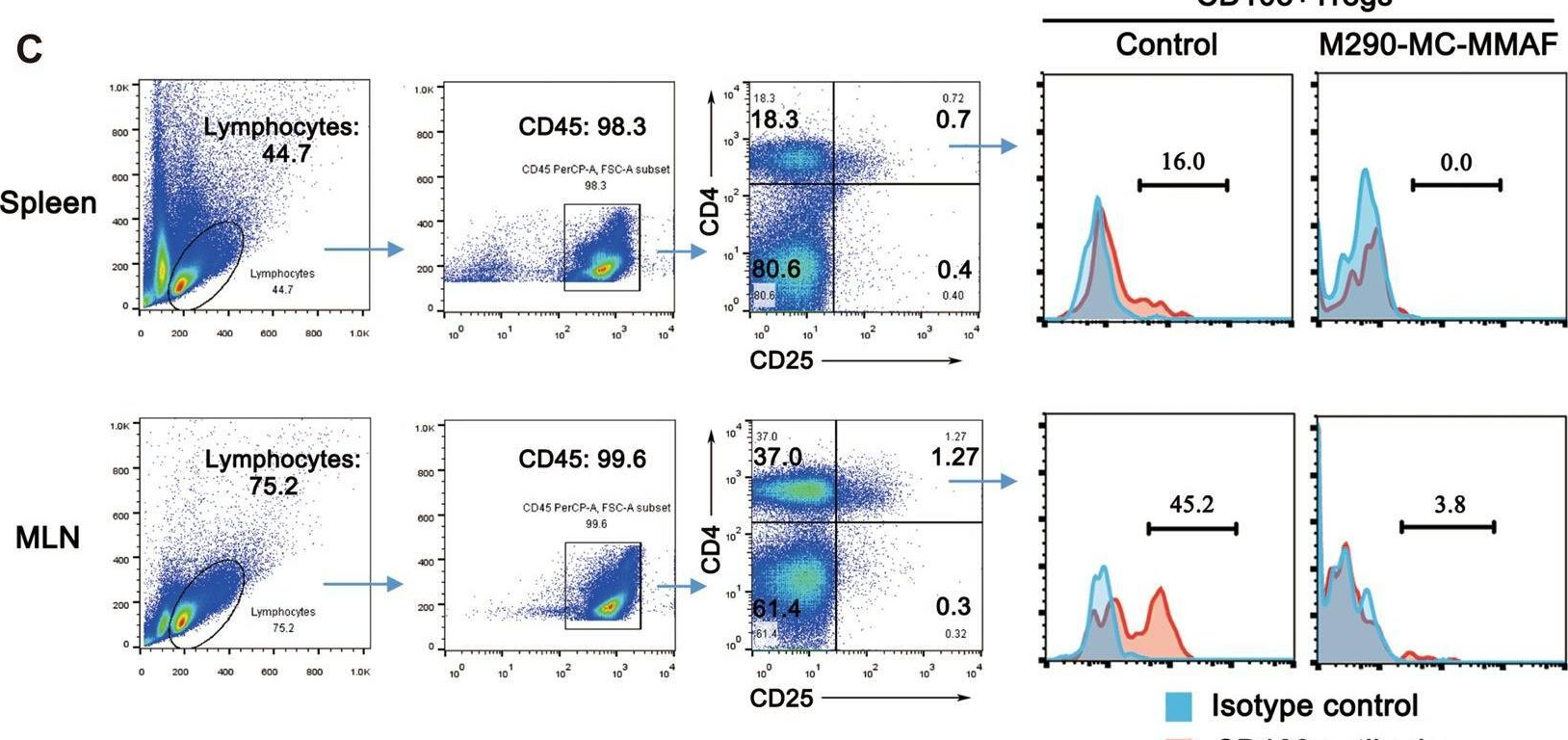
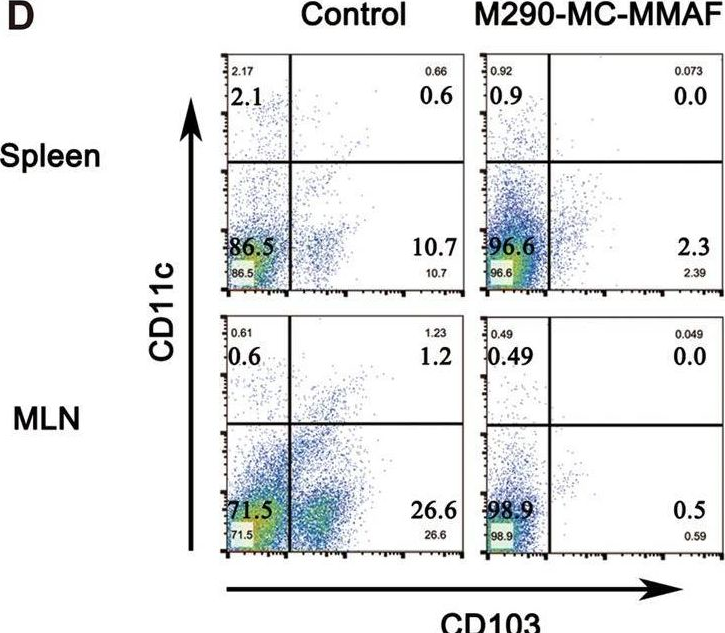
Mang, Y., et al (2015). "Efficient elimination of CD103-expressing cells by anti-CD103 antibody drug conjugates in immunocompetent mice" Int Immunopharmacol 24(1): 119-127.
PubMed
CD103 plays an important role in the destruction of islet allografts, and previous studies found that a CD103 immunotoxin (M290-Saporin, or M290-SAP) promoted the long-term survival of pancreatic islet allografts. However, systemic toxicity to the host and the bystander effects of M290-SAP obscure the underlying mechanisms of action and restrict its clinical applications. To overcome these shortcomings, anti-CD103 M290 was conjugated to different cytotoxic agents through cleavable or uncleavable linkages to form three distinct antibody-drug conjugates (ADCs): M290-MC-vc-PAB-MMAE, M290-MC-MMAF, and M290-MCC-DM1. The drug-to-antibody ratio (DAR) and the purity of the ADCs were determined by HIC-HPLC and SEC-HPLC, respectively. The binding characteristics, internalization and cytotoxicity of M290 and the corresponding ADCs were evaluated in vitro. The cell depletion efficacies of the various M290-ADCs against CD103-positive cells were then evaluated in vivo. The M290-ADCs maintained the initial binding affinity for the CD103-positive cell surface antigen and then quickly internalized the CD103-positive cell. Surprisingly, all M290-ADCs potently depleted CD103-positive cells in vivo, with high specificity and reduced toxicity. Our findings show that M290-ADCs have potent and selective depletion effects on CD103-expressing cells in immunocompetent mice. These data indicate that M290-ADCs could potentially serve as a therapeutic intervention to block the CD103/E-cadherin pathway.
In vivo CD103 neutralization
Mock, J. R., et al (2014). "Foxp3+ regulatory T cells promote lung epithelial proliferation" Mucosal Immunol 7(6): 1440-1451.
PubMed
Acute respiratory distress syndrome (ARDS) causes significant morbidity and mortality each year. There is a paucity of information regarding the mechanisms necessary for ARDS resolution. Foxp3(+) regulatory T cells (Foxp3(+) T(reg) cells) have been shown to be an important determinant of resolution in an experimental model of lung injury. We demonstrate that intratracheal delivery of endotoxin (lipopolysaccharide) elicits alveolar epithelial damage from which the epithelium undergoes proliferation and repair. Epithelial proliferation coincided with an increase in Foxp3(+) T(reg) cells in the lung during the course of resolution. To dissect the role that Foxp3(+) T(reg) cells exert on epithelial proliferation, we depleted Foxp3(+) T(reg) cells, which led to decreased alveolar epithelial proliferation and delayed lung injury recovery. Furthermore, antibody-mediated blockade of CD103, an integrin, which binds to epithelial expressed E-cadherin decreased Foxp3(+) T(reg) numbers and decreased rates of epithelial proliferation after injury. In a non-inflammatory model of regenerative alveologenesis, left lung pneumonectomy, we found that Foxp3(+) T(reg) cells enhanced epithelial proliferation. Moreover, Foxp3(+) T(reg) cells co-cultured with primary type II alveolar cells (AT2) directly increased AT2 cell proliferation in a CD103-dependent manner. These studies provide evidence of a new and integral role for Foxp3(+) T(reg) cells in repair of the lung epithelium.
In vivo CD103 neutralization
Sandoval, F., et al (2013). "Mucosal imprinting of vaccine-induced CD8(+) T cells is crucial to inhibit the growth of mucosal tumors" Sci Transl Med 5(172): 172ra120.
PubMed
Although many human cancers are located in mucosal sites, most cancer vaccines are tested against subcutaneous tumors in preclinical models. We therefore wondered whether mucosa-specific homing instructions to the immune system might influence mucosal tumor outgrowth. We showed that the growth of orthotopic head and neck or lung cancers was inhibited when a cancer vaccine was delivered by the intranasal mucosal route but not the intramuscular route. This antitumor effect was dependent on CD8(+) T cells. Indeed, only intranasal vaccination elicited mucosal-specific CD8(+) T cells expressing the mucosal integrin CD49a. Blockade of CD49a decreased intratumoral CD8(+) T cell infiltration and the efficacy of cancer vaccine on mucosal tumor. We then showed that after intranasal vaccination, dendritic cells from lung parenchyma, but not those from spleen, induced the expression of CD49a on cocultured specific CD8(+) T cells. Tumor-infiltrating lymphocytes from human mucosal lung cancer also expressed CD49a, which supports the relevance and possible extrapolation of these results in humans. We thus identified a link between the route of vaccination and the induction of a mucosal homing program on induced CD8(+) T cells that controlled their trafficking. Immunization route directly affected the efficacy of the cancer vaccine to control mucosal tumors.
Product Citations
-
-
Endocrinology and Physiology
-
Immunology and Microbiology
Lung CD103+dendritic cells and Clec9a signaling are required for neonatal hyperoxia-induced inflammatory responses to rhinovirus infection.
In American Journal of Physiology - Lung Cellular and Molecular Physiology on 1 February 2021 by Cui, T. X., Fulton, C. T., et al.
PubMed
Premature infants, especially those with bronchopulmonary dysplasia (BPD), develop recurrent severe respiratory viral illnesses. We have shown that hyperoxic exposure of immature mice, a model of BPD, increases lung IL-12-producing Clec9a+ CD103+ dendritic cells (DCs), pro-inflammatory responses, and airway hyperreactivity following rhinovirus (RV) infection. However, the requirement for CD103+ DCs and Clec9a, a DAMP receptor that binds necrotic cell cytoskeletal filamentous actin (F-actin), for RV-induced inflammatory responses has not been demonstrated. To test this, 2-day-old C57BL/6J, CD103+ DC-deficient Batf3-/- or Clec9agfp-/- mice were exposed to normoxia or hyperoxia for 14 days. Also, selected mice were treated with neutralizing antibody against CD103. Immediately after hyperoxia, the mice were inoculated with RV intranasally. We found that compared with wild-type mice, hyperoxia-exposed Batf3-/- mice showed reduced levels of IL-12p40, IFN-γ, and TNF-α, fewer IFN-γ-producing CD4+ T cells, and decreased airway responsiveness following RV infection. Similar effects were observed in anti-CD103-treated and Clec9agfp-/- mice. Furthermore, hyperoxia increased airway dead cell number and extracellular F-actin levels. Finally, studies in preterm infants with respiratory distress syndrome showed that tracheal aspirate CLEC9A expression positively correlated with IL12B expression, consistent with the notion that CLEC9A+ cells are responsible for IL-12 production in humans as well as mice. We conclude that CD103+ DCs and Clec9a are required for hyperoxia-induced pro-inflammatory responses to RV infection. In premature infants, Clec9a-mediated activation of CD103+ DCs may promote pro-inflammatory responses to viral infection, thereby driving respiratory morbidity.
-
-
-
Cancer Research
-
Immunology and Microbiology
Different tumour-resident memory T-cell subsets regulate responses to anti-PD-1 and anti-CTLA-4 cancer immunotherapies.
In Nat Commun on 1 July 2025 by Damei, I., Caidi, A., et al.
PubMed
The involvement of tumour-resident memory T (TRM) cells in responses to immune checkpoint inhibitors remains unclear. Here, we show that while CD103+CD8 TRM cells are involved in response to PD-1 blockade, CD49a+CD4 TRM cells are required for the response to anti-CTLA-4. Using preclinical mouse models, we demonstrate that the benefits of anti-PD-1 treatment are compromised in animals challenged with anti-CD8 and anti-CD103 blocking antibodies. By contrast, the benefits of anti-CTLA-4 are decreased by anti-CD4 and anti-CD49a neutralizing antibodies. Single-cell RNA sequencing on tumour-infiltrating T-lymphocytes (TIL) reveals a CD49a+CD4 TRM signature, enriched in Ctla-4 transcripts, exacerbated upon anti-CTLA-4. CTLA-4 blockade expands CD49a+CD4 TRM cells and increases tumour-specific CD4-TIL-mediated cytotoxicity. A CD49a+CD4 TRM signature enriched in CTLA-4 and cytotoxicity-linked transcripts is also identified in human TILs. Multiplex immunohistochemistry in a cohort of anti-CTLA-4-plus-anti-PD-1-treated melanomas reveals an increase in CD49a+CD4 T-cell density in pre-treatment tumours, which correlates with higher rates of patient progression-free survival. Thus, CD49a+CD4 TRM cells may correspond to a predictive biomarker of response to combined immunotherapy.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Development of 89Zr-anti-CD103 PET imaging for non-invasive assessment of cancer reactive T cell infiltration.
In J Immunother Cancer on 1 December 2022 by Kol, A., Fan, X., et al.
PubMed
CD103, an integrin specifically expressed on the surface of cancer-reactive T cells, is significantly increased during successful immunotherapy across human malignancies. In this study, we describe the generation and zirconium-89 (89Zr) radiolabeling of monoclonal antibody (mAb) clones that specifically recognize human CD103 for non-invasive immune positron-emission tomography (PET) imaging of T cell infiltration as potential biomarker for effective anticancer immune responses.
-
-
-
Immunology and Microbiology
SMAD4 TGF-β-independent function preconditions naive CD8+ T cells to prevent severe chronic intestinal inflammation.
In J Clin Invest on 15 April 2022 by Igalouzene, R., Hernández-Vargas, H., et al.
PubMed
SMAD4, a mediator of TGF-β signaling, plays an important role in T cells to prevent inflammatory bowel disease (IBD). However, the precise mechanisms underlying this control remain elusive. Using both genetic and epigenetic approaches, we revealed an unexpected mechanism by which SMAD4 prevents naive CD8+ T cells from becoming pathogenic for the gut. Prior to the engagement of the TGF-β receptor, SMAD4 restrains the epigenetic, transcriptional, and functional landscape of the TGF-β signature in naive CD8+ T cells. Mechanistically, prior to TGF-β signaling, SMAD4 binds to promoters and enhancers of several TGF-β target genes, and by regulating histone deacetylation, suppresses their expression. Consequently, regardless of a TGF-β signal, SMAD4 limits the expression of TGF-β negative feedback loop genes, such as Smad7 and Ski, and likely conditions CD8+ T cells for the immunoregulatory effects of TGF-β. In addition, SMAD4 ablation conferred naive CD8+ T cells with both a superior survival capacity, by enhancing their response to IL-7, as well as an enhanced capacity to be retained within the intestinal epithelium, by promoting the expression of Itgae, which encodes the integrin CD103. Accumulation, epithelial retention, and escape from TGF-β control elicited chronic microbiota-driven CD8+ T cell activation in the gut. Hence, in a TGF-β-independent manner, SMAD4 imprints a program that preconditions naive CD8+ T cell fate, preventing IBD.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
The SKI proto-oncogene restrains the resident CD103+CD8+ T cell response in viral clearance.
In Cell Mol Immunol on 1 October 2021 by Wu, B., Zhang, G., et al.
PubMed
Acute viral infection causes illness and death. In addition, an infection often results in increased susceptibility to a secondary infection, but the mechanisms behind this susceptibility are poorly understood. Since its initial identification as a marker for resident memory CD8+ T cells in barrier tissues, the function and regulation of CD103 integrin (encoded by ITGAE gene) have been extensively investigated. Nonetheless, the function and regulation of the resident CD103+CD8+ T cell response to acute viral infection remain unclear. Although TGFβ signaling is essential for CD103 expression, the precise molecular mechanism behind this regulation is elusive. Here, we reveal a TGFβ-SKI-Smad4 pathway that critically and specifically directs resident CD103+CD8+ T cell generation for protective immunity against primary and secondary viral infection. We found that resident CD103+CD8+ T cells are abundant in both lymphoid and nonlymphoid tissues from uninfected mice. CD103 acts as a costimulation signal to produce an optimal antigenic CD8+ T cell response to acute viral infection. There is a reduction in resident CD103+CD8+ T cells following primary infection that results in increased susceptibility of the host to secondary infection. Intriguingly, CD103 expression inversely and specifically correlates with SKI proto-oncogene (SKI) expression but not R-Smad2/3 activation. Ectopic expression of SKI restricts CD103 expression in CD8+ T cells in vitro and in vivo to hamper viral clearance. Mechanistically, SKI is recruited to the Itgae loci to directly suppress CD103 transcription by regulating histone acetylation in a Smad4-dependent manner. Our study therefore reveals that resident CD103+CD8+ T cells dictate protective immunity during primary and secondary infection. Interfering with SKI function may amplify the resident CD103+CD8+ T cell response to promote protective immunity.
-
-
-
Blocking experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Integrin-αV-mediated activation of TGF-β regulates anti-tumour CD8 T cell immunity and response to PD-1 blockade.
In Nat Commun on 1 September 2021 by Malenica, I., Adam, J., et al.
PubMed
TGF-β is secreted in the tumour microenvironment in a latent, inactive form bound to latency associated protein and activated by the integrin αV subunit. The activation of latent TGF-β by cancer-cell-expressed αV re-shapes the tumour microenvironment, and this could affect patient responses to PD-1-targeting therapy. Here we show, using multiplex immunofluorescence staining in cohorts of anti-PD-1 and anti-PD-L1-treated lung cancer patients, that decreased expression of cancer cell αV is associated with improved immunotherapy-related, progression-free survival, as well as with an increased density of CD8+CD103+ tumour-infiltrating lymphocytes. Mechanistically, tumour αV regulates CD8 T cell recruitment, induces CD103 expression on activated CD8+ T cells and promotes their differentiation to granzyme B-producing CD103+CD69+ resident memory T cells via autocrine TGF-β signalling. Thus, our work provides the underlying principle of targeting cancer cell αV for more efficient PD-1 checkpoint blockade therapy.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Dual targeting of lymphocyte homing and retention through α4β7 and αEβ7 inhibition in inflammatory bowel disease.
In Cell Rep Med on 17 August 2021 by Dai, B., Hackney, J. A., et al.
PubMed
Anti-integrins are therapeutically effective for inflammatory bowel disease, yet the relative contribution of α4β7 and αEβ7 to gut lymphocyte trafficking is not fully elucidated. Here, we evaluate the effect of α4β7 and αEβ7 blockade using a combination of murine models of gut trafficking and longitudinal gene expression analysis in etrolizumab-treated patients with Crohn's disease (CD). Dual blockade of α4β7 and αEβ7 reduces CD8+ T cell accumulation in the gut to a greater extent than blockade of either integrin alone. Anti-αEβ7 reduces epithelial:T cell interactions and promotes egress of activated T cells from the mucosa into lymphatics. Inflammatory gene expression is greater in human intestinal αEβ7+ T cells. Etrolizumab-treated patients with CD display a treatment-specific reduction in inflammatory and cytotoxic intraepithelial lymphocytes (IEL) genes. Concurrent blockade of α4β7 and αEβ7 promotes reduction of cytotoxic IELs and inflammatory T cells in the gut mucosa through a stepwise inhibition of intestinal tissue entry and retention.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Hypoxia-inducible factor activity promotes antitumor effector function and tissue residency by CD8+ T cells.
In J Clin Invest on 1 April 2021 by Liikanen, I., Lauhan, C., et al.
PubMed
Adoptive T cell therapies (ACTs) hold great promise in cancer treatment, but low overall response rates in patients with solid tumors underscore remaining challenges in realizing the potential of this cellular immunotherapy approach. Promoting CD8+ T cell adaptation to tissue residency represents an underutilized but promising strategy to improve tumor-infiltrating lymphocyte (TIL) function. Here, we report that deletion of the HIF negative regulator von Hippel-Lindau (VHL) in CD8+ T cells induced HIF-1α/HIF-2α-dependent differentiation of tissue-resident memory-like (Trm-like) TILs in mouse models of malignancy. VHL-deficient TILs accumulated in tumors and exhibited a core Trm signature despite an exhaustion-associated phenotype, which led to retained polyfunctionality and response to αPD-1 immunotherapy, resulting in tumor eradication and protective tissue-resident memory. VHL deficiency similarly facilitated enhanced accumulation of chimeric antigen receptor (CAR) T cells with a Trm-like phenotype in tumors. Thus, HIF activity in CD8+ TILs promotes accumulation and antitumor activity, providing a new strategy to enhance the efficacy of ACTs.
-
-
-
Endocrinology and Physiology
-
Immunology and Microbiology
Lung CD103+dendritic cells and Clec9a signaling are required for neonatal hyperoxia-induced inflammatory responses to rhinovirus infection.
In Am J Physiol Lung Cell Mol Physiol on 1 February 2021 by Cui, T. X., Fulton, C. T., et al.
PubMed
Premature infants, especially those with bronchopulmonary dysplasia (BPD), develop recurrent severe respiratory viral illnesses. We have shown that hyperoxic exposure of immature mice, a model of BPD, increases lung IL-12-producing Clec9a+ CD103+ dendritic cells (DCs), pro-inflammatory responses, and airway hyperreactivity following rhinovirus (RV) infection. However, the requirement for CD103+ DCs and Clec9a, a DAMP receptor that binds necrotic cell cytoskeletal filamentous actin (F-actin), for RV-induced inflammatory responses has not been demonstrated. To test this, 2-day-old C57BL/6J, CD103+ DC-deficient Batf3-/- or Clec9agfp-/- mice were exposed to normoxia or hyperoxia for 14 days. Also, selected mice were treated with neutralizing antibody against CD103. Immediately after hyperoxia, the mice were inoculated with RV intranasally. We found that compared with wild-type mice, hyperoxia-exposed Batf3-/- mice showed reduced levels of IL-12p40, IFN-γ, and TNF-α, fewer IFN-γ-producing CD4+ T cells, and decreased airway responsiveness following RV infection. Similar effects were observed in anti-CD103-treated and Clec9agfp-/- mice. Furthermore, hyperoxia increased airway dead cell number and extracellular F-actin levels. Finally, studies in preterm infants with respiratory distress syndrome showed that tracheal aspirate CLEC9A expression positively correlated with IL12B expression, consistent with the notion that CLEC9A+ cells are responsible for IL-12 production in humans as well as mice. We conclude that CD103+ DCs and Clec9a are required for hyperoxia-induced pro-inflammatory responses to RV infection. In premature infants, Clec9a-mediated activation of CD103+ DCs may promote pro-inflammatory responses to viral infection, thereby driving respiratory morbidity.
-
-
γδ intraepithelial lymphocytes facilitate pathological epithelial cell shedding via CD103-mediated granzyme release
In bioRxiv on 21 January 2021 by Hu, M. D., Golovchenko, N. B., et al.
-
-
Conjugation assay
-
Cell Biology
-
Conjugation assay
An anti-CD103 antibody-drug conjugate prolongs the survival of pancreatic islet allografts in mice.
In Cell Death Dis on 30 September 2019 by Xue, D., Liu, P., et al.
PubMed
CD103 mediates T-cell infiltration and organ allograft rejection, and depletion of CD103-expressing cells is a promising therapeutic strategy for allograft intolerance. Recently, we verified that M290-MC-MMAF, an anti-CD103 antibody-drug conjugate, potently eliminates CD103-positive cells in vivo, with high specificity and minimal toxicity. However, the contribution of M290-MC-MMAF to blocking the CD103/E-cadherin pathway involved in transplant rejection remains unclear. Herein, we examined the impact of systemic administration of M290-MC-MMAF on allografts in an islet transplantation model. M290-MC-MMAF treatment maintained the long-term survival of islet allografts (>60 days) compared to mock injection or unconjugated M290 antibody treatment (<18 days). The change was associated with a decrease in CD103+CD8+ effector T cells and an increase in CD4+CD25+ regulatory T cells. CD103+CD8+ effector T-cell transfer or CD4+CD25+ regulatory T-cell depletion resulted in a rapid loss of allografts in long-surviving islet hosts. Moreover, M290-MC-MMAF treatment reduced IL-4, IL-6, and TNF-α expression levels and increased IL-10 expression in the grafts, which presented an immunosuppressive cytokine profile. In conclusion, targeting CD103 with M290-MC-MMAF induced immunosuppression and prolonged the survival of pancreatic islet allografts in mice, indicating the potential clinical value of M290-MC-MMAF in therapeutic interventions for allograft rejection.
-
-
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Response to Programmed Cell Death-1 Blockade in a Murine Melanoma Syngeneic Model Requires Costimulation, CD4, and CD8 T Cells.
In Cancer Immunol Res on 1 October 2016 by Homet Moreno, B., Zaretsky, J. M., et al.
PubMed
The programmed cell death protein 1 (PD-1) limits effector T-cell functions in peripheral tissues, and its inhibition leads to clinical benefit in different cancers. To better understand how PD-1 blockade therapy modulates the tumor-host interactions, we evaluated three syngeneic murine tumor models, the BRAFV600E-driven YUMM1.1 and YUMM2.1 melanomas, and the carcinogen-induced murine colon adenocarcinoma MC38. The YUMM cell lines were established from mice with melanocyte-specific BRAFV600E mutation and PTEN loss (BRAFV600E/PTEN-/-). Anti-PD-1 or anti-PD-L1 therapy engendered strong antitumor activity against MC38 and YUMM2.1, but not YUMM1.1. PD-L1 expression did not differ between the three models at baseline or upon interferon stimulation. Whereas mutational load was high in MC38, it was lower in both YUMM models. In YUMM2.1, the antitumor activity of PD-1 blockade had a critical requirement for both CD4 and CD8 T cells, as well as CD28 and CD80/86 costimulation, with an increase in CD11c+CD11b+MHC-IIhigh dendritic cells and tumor-associated macrophages in the tumors after PD-1 blockade. Compared with YUMM1.1, YUMM2.1 exhibited a more inflammatory profile by RNA sequencing analysis, with an increase in expression of chemokine-trafficking genes that are related to immune cell recruitment and T-cell priming. In conclusion, response to PD-1 blockade therapy in tumor models requires CD4 and CD8 T cells and costimulation that is mediated by dendritic cells and macrophages. Cancer Immunol Res; 4(10); 845-57. ©2016 AACR.
-
-
-
In vivo experiments
-
In vivo experiments
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Mucosal imprinting of vaccine-induced CD8⁺ T cells is crucial to inhibit the growth of mucosal tumors.
In Sci Transl Med on 13 February 2013 by Sandoval, F., Terme, M., et al.
PubMed
Although many human cancers are located in mucosal sites, most cancer vaccines are tested against subcutaneous tumors in preclinical models. We therefore wondered whether mucosa-specific homing instructions to the immune system might influence mucosal tumor outgrowth. We showed that the growth of orthotopic head and neck or lung cancers was inhibited when a cancer vaccine was delivered by the intranasal mucosal route but not the intramuscular route. This antitumor effect was dependent on CD8⁺ T cells. Indeed, only intranasal vaccination elicited mucosal-specific CD8⁺ T cells expressing the mucosal integrin CD49a. Blockade of CD49a decreased intratumoral CD8⁺ T cell infiltration and the efficacy of cancer vaccine on mucosal tumor. We then showed that after intranasal vaccination, dendritic cells from lung parenchyma, but not those from spleen, induced the expression of CD49a on cocultured specific CD8⁺ T cells. Tumor-infiltrating lymphocytes from human mucosal lung cancer also expressed CD49a, which supports the relevance and possible extrapolation of these results in humans. We thus identified a link between the route of vaccination and the induction of a mucosal homing program on induced CD8⁺ T cells that controlled their trafficking. Immunization route directly affected the efficacy of the cancer vaccine to control mucosal tumors.
-