InVivoMAb anti-mouse CD3ε
Product Details
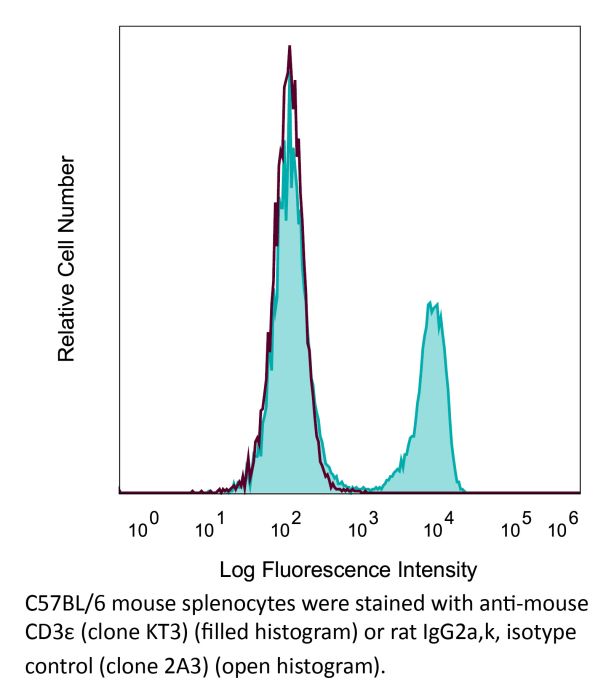
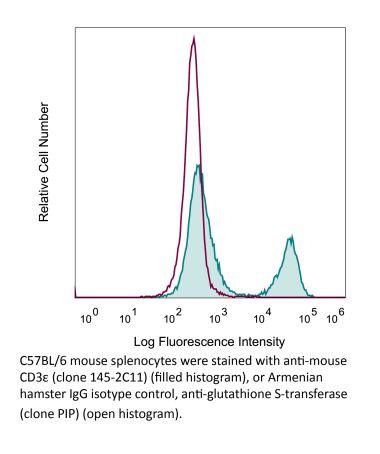
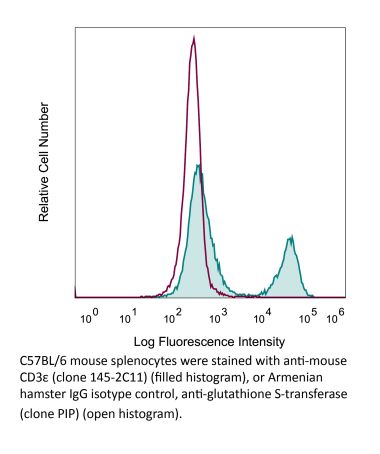
The KT3 monoclonal antibody reacts with mouse CD3ε, a 20 kDa transmembrane cell-surface protein that belongs to the immunoglobulin superfamily. CD3ε is one of five polypeptide chains that combine to form the TCR complex. CD3ε is expressed on T lymphocytes, NK-T cells, and to varying degrees on developing thymocytes. CD3 plays roles in TCR signaling, T lymphocyte activation, and antigen recognition. The KT3 antibody has been shown to induce T lymphocyte activation via binding and stimulating the TCR.Specifications
| Isotype | Rat IgG2a |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | CBAT6 mouse thymocytes |
| Reported Applications |
in vitro T cell negative selection in vitro T cell stimulation/activation Immunofluorescence |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687740 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vitro T cell negative selection
Reuter, A., et al. (2015). "Criteria for dendritic cell receptor selection for efficient antibody-targeted vaccination" J Immunol 194(6): 2696-2705. PubMed
Ab-targeted vaccination involves targeting a receptor of choice expressed by dendritic cells (DCs) with Ag-coupled Abs. Currently, there is little consensus as to which criteria determine receptor selection to ensure superior Ag presentation and immunity. In this study, we investigated parameters of DC receptor internalization and determined how they impact Ag presentation outcomes. First, using mixed bone marrow chimeras, we established that Ag-targeted, but not nontargeted, DCs are responsible for Ag presentation in settings of Ab-targeted vaccination in vivo. Next, we analyzed parameters of DEC205 (CD205), Clec9A, CD11c, CD11b, and CD40 endocytosis and obtained quantitative measurements of internalization speed, surface turnover, and delivered Ag load. Exploiting these parameters in MHC class I (MHC I) and MHC class II (MHC II) Ag presentation assays, we showed that receptor expression level, proportion of surface turnover, or speed of receptor internalization did not impact MHC I or MHC II Ag presentation efficiency. Furthermore, the Ag load delivered to DCs did not correlate with the efficiency of MHC I or MHC II Ag presentation. In contrast, targeting Ag to CD8(+) or CD8(-) DCs enhanced MHC I or MHC II Ag presentation, respectively. Therefore, receptor expression levels, speed of internalization, and/or the amount of Ag delivered can be excluded as major determinants that dictate Ag presentation efficiency in setting of Ab-targeted vaccination.
in vitro T cell stimulation/activation
Tone, Y., et al. (2014). "Gene expression in the Gitr locus is regulated by NF-kappaB and Foxp3 through an enhancer" J Immunol 192(8): 3915-3924. PubMed
Glucocorticoid-induced TNFR (Gitr) and Ox40, two members of the TNFR superfamily, play important roles in regulating activities of effector and regulatory T cells (Treg). Their gene expression is induced by T cell activation and further upregulated in Foxp3+ Treg. Although the role of Foxp3 as a transcriptional repressor in Treg is well established, the mechanisms underlying Foxp3-mediated transcriptional upregulation remain poorly understood. This transcription factor seems to upregulate expression not only of Gitr and Ox40, but also other genes, including Ctla4, Il35, Cd25, all critical to Treg function. To investigate how Foxp3 achieves such upregulation, we analyzed its activity on Gitr and Ox40 genes located within a 15.1-kb region. We identified an enhancer located downstream of the Gitr gene, and both Gitr and Ox40 promoter activities were shown to be upregulated by the NF-kappaB-mediated enhancer activity. We also show, using the Gitr promoter, that the enhancer activity was further upregulated in conjunction with Foxp3. Foxp3 appears to stabilize NF-kappaB p50 binding by anchoring it to the enhancer, thereby enabling local accumulation of transcriptional complexes containing other members of the NF-kappaB and IkappaB families. These findings may explain how Foxp3 can activate expression of certain genes while suppressing others.
Immunofluorescence
Thawer, S. G., et al. (2014). "Lung-resident CD4(+) T cells are sufficient for IL-4Ralpha-dependent recall immunity to Nippostrongylus brasiliensis infection" Mucosal Immunol 7(2): 239-248. PubMed
Immunity to Nippostrongylus brasiliensis reinfection requires pulmonary CD4(+) T-cell responses. We examined whether secondary lymphoid recruited or pre-existing lung CD4(+) T-cell populations coordinated this immunity. To do this, we blocked T-cell egress from lymph nodes using Fingolimod (FTY720). This impaired host ability to resolve a primary infection but did not change effectiveness of recall immunity. Associated with this effective recall immunity was the expansion and T helper type 2 polarization of a pre-existing pulmonary CD4(+) T-cell population. LTbetaR-Ig (lymphotoxin beta-receptor fusion protein)-mediated disruption of stromal cell organization of immune cells did not disrupt this recall immunity, suggesting that protection was mediated by a pulmonary interstitial residing CD4(+) T-cell population. Adoptive transfer of N. brasiliensis-experienced pulmonary CD4(+) T cells from FTY720-treated wild-type or T-cell interleukin (IL)-4Ralpha-deficient mice demonstrated protection to be IL-4Ralpha dependent. These results show that pre-existing CD4(+) T cells can drive effective recall immunity to N. brasiliensis infection independently of T-cell recruitment from secondary lymphoid organs.
in vitro T cell negative selection
Sathe, P., et al. (2014). "Lymphoid tissue and plasmacytoid dendritic cells and macrophages do not share a common macrophage-dendritic cell-restricted progenitor" Immunity 41(1): 104-115. PubMed
The relationship between dendritic cells (DCs) and macrophages is often debated. Here we ask whether steady-state, lymphoid-tissue-resident conventional DCs (cDCs), plasmacytoid DCs (pDCs), and macrophages share a common macrophage-DC-restricted precursor (MDP). Using new clonal culture assays combined with adoptive transfer, we found that MDP fractions isolated by previous strategies are dominated by precursors of macrophages and monocytes, include some multipotent precursors of other hematopoietic lineages, but contain few precursors of resident cDCs and pDCs and no detectable common precursors restricted to these DC types and macrophages. Overall we find no evidence for a common restricted MDP leading to both macrophages and FL-dependent, resident cDCs and pDCs.
in vitro T cell negative selection
Moffat, J. M., et al. (2013). "Hepatitis B virus-like particles access major histocompatibility class I and II antigen presentation pathways in primary dendritic cells" Vaccine 31(18): 2310-2316. PubMed
Virus-like particles (VLPs) represent high density displays of viral proteins that efficiently trigger immunity. VLPs composed of the small hepatitis B virus envelope protein (HBsAgS) are useful vaccine platforms that induce humoral and cellular immune responses. Notably, however, some studies suggest HBsAgS VLPs impair dendritic cell (DC) function. Here we investigated HBsAgS VLP interaction with DC subsets and antigen access to major histocompatibility complex (MHC) class I and II antigen presentation pathways in primary DCs. HBsAgS VLPs impaired plasmacytoid DC (pDC) interferon alpha (IFNalpha) production in response to CpG in vitro, but did not alter conventional DC (cDC) or pDC phenotype when administered in vivo. To assess cellular immune responses, HBsAgS VLPs were generated containing the ovalbumin (OVA) model epitopes OVA(257-264) and OVA(323-339) to access MHCI and MHCII antigen presentation pathways, respectively; both in vitro and following immunisation in vivo. HBsAgS VLP-OVA(257-264) elicited CTL responses in vivo that were not enhanced by inclusion of an additional MHCII helper epitope. HBsAgS VLP-OVA(257-264) administered in vivo was cross-presented by CD8(+) DCs, but not CD8(-) DCs. Therefore, HBsAgS VLPs can deliver antigen to both MHCI and MHCII antigen presentation pathways in primary DCs and promote cytotoxic and helper T cell priming despite their suppressive effect on pDCs.
- Cancer Research,
- Immunology and Microbiology
Myeloid cell subsets that express latency-associated peptide promote cancer growth by modulating T cells.
In IScience on 19 November 2021 by Gabriely, G., Ma, D., et al.
PubMed
Myeloid suppressor cells promote tumor growth by a variety of mechanisms which are not fully characterized. We identified myeloid cells (MCs) expressing the latency-associated peptide (LAP) of TGF-β on their surface and LAPHi MCs that stimulate Foxp3+ Tregs while inhibiting effector T cell proliferation and function. Blocking TGF-β inhibits the tolerogenic ability of LAPHi MCs. Furthermore, adoptive transfer of LAPHi MCs promotes Treg accumulation and tumor growth in vivo. Conversely, anti-LAP antibody, which reduces LAPHi MCs, slows cancer progression. Single-cell RNA-Seq analysis on tumor-derived immune cells revealed LAPHi dominated cell subsets with distinct immunosuppressive signatures, including those with high levels of MHCII and PD-L1 genes. Analogous to mice, LAP is expressed on myeloid suppressor cells in humans, and these cells are increased in glioma patients. Thus, our results identify a previously unknown function by which LAPHi MCs promote tumor growth and offer therapeutic intervention to target these cells in cancer.© 2021 The Authors.
- In Vitro,
- FC/FACS,
- Mus musculus (House mouse),
- Biochemistry and Molecular biology,
- Cell Biology,
- Immunology and Microbiology
The Transcription Factor Bhlhe40 Programs Mitochondrial Regulation of Resident CD8+ T Cell Fitness and Functionality.
In Immunity on 17 September 2019 by Li, C., Zhu, B., et al.
PubMed
Tissue-resident memory CD8+ T (Trm) cells share core residency gene programs with tumor-infiltrating lymphocytes (TILs). However, the transcriptional, metabolic, and epigenetic regulation of Trm cell and TIL development and function is largely undefined. Here, we found that the transcription factor Bhlhe40 was specifically required for Trm cell and TIL development and polyfunctionality. Local PD-1 signaling inhibited TIL Bhlhe40 expression, and Bhlhe40 was critical for TIL reinvigoration following anti-PD-L1 blockade. Mechanistically, Bhlhe40 sustained Trm cell and TIL mitochondrial fitness and a functional epigenetic state. Building on these findings, we identified an epigenetic and metabolic regimen that promoted Trm cell and TIL gene signatures associated with tissue residency and polyfunctionality. This regimen empowered the anti-tumor activity of CD8+ T cells and possessed therapeutic potential even at an advanced tumor stage in mouse models. Our results provide mechanistic insights into the local regulation of Trm cell and TIL function. Copyright © 2019 Elsevier Inc. All rights reserved.