InVivoMAb anti-mouse IL-1 R (CD121a)
Product Details
The JAMA-147 monoclonal antibody reacts with mouse IL-1 receptor (IL-1 R) type 1 also known as CD121a. IL-1 R is an 80 kDa transmembrane glycoprotein and a member of the immunoglobulin superfamily. The receptor is expressed on T cells, thymocytes, dendritic cells, fibroblasts, vascular endothelial cells, epithelial cells and neural cells. IL-1 R type 1 can bind both IL-1α and IL-1β. Upon ligand binding the type I receptor mediates all the known IL-1 biological responses.Specifications
| Isotype | Armenian Hamster IgG, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 6.0T Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Extracellular domain of mouse IL-1 R type 1 |
| Reported Applications |
in vivo IL-1 R blockade in vitro IL-1 R blockade |
| Formulation |
PBS, pH 6.0 0.01% Tween Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2661843 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo IL-1 R blockade
Singh, S., et al. (2021). "IL-1alpha Mediates Innate and Acquired Resistance to Immunotherapy in Melanoma" J Immunol 206(8): 1966-1975. PubMed
Inflammation has long been associated with cancer initiation and progression; however, how inflammation causes immune suppression in the tumor microenvironment and resistance to immunotherapy is not well understood. In this study, we show that both innate proinflammatory cytokine IL-1alpha and immunotherapy-induced IL-1alpha make melanoma resistant to immunotherapy. In a mouse melanoma model, we found that tumor size was inversely correlated with response to immunotherapy. Large tumors had higher levels of IL-1alpha, Th2 cytokines, polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), and regulatory T cells but lower levels of IL-12, Th1 cytokines, and activated T cells. We found that therapy with adenovirus-encoded CD40L (rAd.CD40L) increased tumor levels of IL-1alpha and PMN-MDSCs. Blocking the IL-1 signaling pathway significantly decreased rAd.CD40L-induced PMN-MDSCs and their associated PD-L1 expression in the tumor microenvironment and enhanced tumor-specific immunity. Similarly, blocking the IL-1 signaling pathway improved the antimelanoma activity of anti-PD-L1 Ab therapy. Our study suggests that blocking the IL-1alpha signaling pathway may increase the efficacy of immunotherapies against melanoma.
in vivo IL-1 R blockade
Kongsomboonvech, A. K., et al. (2020). "Naïve CD8 T cell IFNγ responses to a vacuolar antigen are regulated by an inflammasome-independent NLRP3 pathway and Toxoplasma gondii ROP5" PLoS Pathog 16(8): e1008327. PubMed
Host resistance to Toxoplasma gondii relies on CD8 T cell IFNγ responses, which if modulated by the host or parasite could influence chronic infection and parasite transmission between hosts. Since host-parasite interactions that govern this response are not fully elucidated, we investigated requirements for eliciting naïve CD8 T cell IFNγ responses to a vacuolar resident antigen of T. gondii, TGD057. Naïve TGD057 antigen-specific CD8 T cells (T57) were isolated from transnuclear mice and responded to parasite-infected bone marrow-derived macrophages (BMDMs) in an antigen-dependent manner, first by producing IL-2 and then IFNγ. T57 IFNγ responses to TGD057 were independent of the parasite’s protein export machinery ASP5 and MYR1. Instead, host immunity pathways downstream of the regulatory Immunity-Related GTPases (IRG), including partial dependence on Guanylate-Binding Proteins, are required. Multiple T. gondii ROP5 isoforms and allele types, including ‘avirulent’ ROP5A from clade A and D parasite strains, were able to suppress CD8 T cell IFNγ responses to parasite-infected BMDMs. Phenotypic variance between clades B, C, D, F, and A strains suggest T57 IFNγ differentiation occurs independently of parasite virulence or any known IRG-ROP5 interaction. Consistent with this, removal of ROP5 is not enough to elicit maximal CD8 T cell IFNγ production to parasite-infected cells. Instead, macrophage expression of the pathogen sensors, NLRP3 and to a large extent NLRP1, were absolute requirements. Other members of the conventional inflammasome cascade are only partially required, as revealed by decreased but not abrogated T57 IFNγ responses to parasite-infected ASC, caspase-1/11, and gasdermin D deficient cells. Moreover, IFNγ production was only partially reduced in the absence of IL-12, IL-18 or IL-1R signaling. In summary, T. gondii effectors and host machinery that modulate parasitophorous vacuolar membranes, as well as NLR-dependent but inflammasome-independent pathways, determine the full commitment of CD8 T cells IFNγ responses to a vacuolar antigen.
in vivo IL-1 R blockade
Allen, B. M., et al. (2020). "Systemic dysfunction and plasticity of the immune macroenvironment in cancer models" Nat Med 26(7): 1125-1134. PubMed
Understanding of the factors governing immune responses in cancer remains incomplete, limiting patient benefit. In this study, we used mass cytometry to define the systemic immune landscape in response to tumor development across five tissues in eight mouse tumor models. Systemic immunity was dramatically altered across models and time, with consistent findings in the peripheral blood of patients with breast cancer. Changes in peripheral tissues differed from those in the tumor microenvironment. Mice with tumor-experienced immune systems mounted dampened responses to orthogonal challenges, including reduced T cell activation during viral or bacterial infection. Antigen-presenting cells (APCs) mounted weaker responses in this context, whereas promoting APC activation rescued T cell activity. Systemic immune changes were reversed with surgical tumor resection, and many were prevented by interleukin-1 or granulocyte colony-stimulating factor blockade, revealing remarkable plasticity in the systemic immune state. These results demonstrate that tumor development dynamically reshapes the composition and function of the immune macroenvironment.
in vivo IL-1 R blockade
Choi, G. E., et al. (2018). "Autophagy deficiency in myeloid cells exacerbates eosinophilic inflammation in chronic rhinosinusitis" J Allergy Clin Immunol 141(3): 938-950 e912. PubMed
BACKGROUND: Eosinophilic inflammation is a major pathologic feature of chronic rhinosinusitis (CRS) and is frequently associated with severe refractory disease. Prostaglandin (PG) D2 levels are increased in patients with CRS, and PGD2 is an important contributing factor to eosinophilic inflammation. Autophagy has a pleiotropic effect on immune responses and disease pathogenesis. Recent studies suggest the potential involvement of autophagy in patients with CRS and the PG pathway. OBJECTIVE: We sought to investigate whether altered function of autophagy is associated with eosinophilic inflammation and dysregulated production of PGD2 in patients with CRS. METHODS: We used myeloid cell-specific deletion of autophagy-related gene 7 (Atg7), which is vital for autophagy, and investigated the effects of impaired autophagy on eosinophilic inflammation in a murine model of eosinophilic chronic rhinosinusitis (ECRS). The effect of autophagy on PGD2 production and gene expression profiles associated with allergy and the PG pathway were assessed. RESULTS: We found that impaired autophagy in myeloid cells aggravated eosinophilia, epithelial hyperplasia, and mucosal thickening in mice with ECRS. This aggravation was associated with gene expression profiles that favor eosinophilic inflammation, TH2 response, mast cell infiltration, and PGD2 dysregulation. Supporting this, PGD2 production was also increased significantly by impaired autophagy. Among other myeloid cells, macrophages were associated with autophagy deficiency, leading to increased IL-1beta levels. Macrophage depletion or blockade of IL-1 receptor led to alleviation of eosinophilic inflammation and sinonasal anatomic abnormalities associated with autophagy deficiency. CONCLUSION: Our results suggest that impaired autophagy in myeloid cells, particularly macrophages, has a causal role in eosinophilic inflammation and ECRS pathogenesis.
Gimblet, C., et al. (2017). "Cutaneous Leishmaniasis Induces a Transmissible Dysbiotic Skin Microbiota that Promotes Skin Inflammation" Cell Host Microbe 22(1): 13-24.e14. PubMed
Skin microbiota can impact allergic and autoimmune responses, wound healing, and anti-microbial defense. We investigated the role of skin microbiota in cutaneous leishmaniasis and found that human patients infected with Leishmania braziliensis develop dysbiotic skin microbiota, characterized by increases in the abundance of Staphylococcus and/or Streptococcus. Mice infected with L. major exhibit similar changes depending upon disease severity. Importantly, this dysbiosis is not limited to the lesion site, but is transmissible to normal skin distant from the infection site and to skin from co-housed naive mice. This observation allowed us to test whether a pre-existing dysbiotic skin microbiota influences disease, and we found that challenging dysbiotic naive mice with L. major or testing for contact hypersensitivity results in exacerbated skin inflammatory responses. These findings demonstrate that a dysbiotic skin microbiota is not only a consequence of tissue stress, but also enhances inflammation, which has implications for many inflammatory cutaneous diseases.
in vivo IL-1 R blockade
Naik, S., et al. (2017). "Inflammatory memory sensitizes skin epithelial stem cells to tissue damage" Nature 550(7677): 475-480. PubMed
The skin barrier is the body’s first line of defence against environmental assaults, and is maintained by epithelial stem cells (EpSCs). Despite the vulnerability of EpSCs to inflammatory pressures, neither the primary response to inflammation nor its enduring consequences are well understood. Here we report a prolonged memory to acute inflammation that enables mouse EpSCs to hasten barrier restoration after subsequent tissue damage. This functional adaptation does not require skin-resident macrophages or T cells. Instead, EpSCs maintain chromosomal accessibility at key stress response genes that are activated by the primary stimulus. Upon a secondary challenge, genes governed by these domains are transcribed rapidly. Fuelling this memory is Aim2, which encodes an activator of the inflammasome. The absence of AIM2 or its downstream effectors, caspase-1 and interleukin-1beta, erases the ability of EpSCs to recollect inflammation. Although EpSCs benefit from inflammatory tuning by heightening their responsiveness to subsequent stressors, this enhanced sensitivity probably increases their susceptibility to autoimmune and hyperproliferative disorders, including cancer.
in vivo IL-1 R blockade
Lin, C. C., et al. (2016). "IL-1-induced Bhlhe40 identifies pathogenic T helper cells in a model of autoimmune neuroinflammation" J Exp Med 213(2): 251-271. PubMed
The features that define autoreactive T helper (Th) cell pathogenicity remain obscure. We have previously shown that Th cells require the transcription factor Bhlhe40 to mediate experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. Here, using Bhlhe40 reporter mice and analyzing both polyclonal and TCR transgenic Th cells, we found that Bhlhe40 expression was heterogeneous after EAE induction, with Bhlhe40-expressing cells displaying marked production of IFN-γ, IL-17A, and granulocyte-macrophage colony-stimulating factor. In adoptive transfer EAE models, Bhlhe40-deficient Th1 and Th17 cells were both nonencephalitogenic. Pertussis toxin (PTX), a classical co-adjuvant for actively induced EAE, promoted IL-1β production by myeloid cells in the draining lymph node and served as a strong stimulus for Bhlhe40 expression in Th cells. Furthermore, PTX co-adjuvanticity was Bhlhe40 dependent. IL-1β induced Bhlhe40 expression in polarized Th17 cells, and Bhlhe40-expressing cells exhibited an encephalitogenic transcriptional signature. In vivo, IL-1R signaling was required for full Bhlhe40 expression by Th cells after immunization. Overall, we demonstrate that Bhlhe40 expression identifies encephalitogenic Th cells and defines a PTX-IL-1-Bhlhe40 pathway active in EAE.
in vitro IL-1 R blockade
Ha, H. L., et al. (2014). "IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms" Proc Natl Acad Sci U S A 111(33): E3422-3431. PubMed
Psoriasis is a chronic inflammatory skin disease characterized by abnormal keratinocyte proliferation and differentiation and by an influx of inflammatory cells. The mechanisms underlying psoriasis in humans and in mouse models are poorly understood, although evidence strongly points to crucial contributions of IL-17 cytokines, which signal via the obligatory adaptor CIKS/Act1. Here we identify critical roles of CIKS/Act1-mediated signaling in imiquimod-induced psoriatic inflammation, a mouse model that shares features with the human disease. We found that IL-17 cytokines/CIKS-mediated signaling into keratinocytes is essential for neutrophilic microabscess formation and contributes to hyperproliferation and markedly attenuated differentiation of keratinocytes, at least in part via direct effects. In contrast, IL-17 cytokines/CIKS-mediated signaling into nonkeratinocytes, particularly into dermal fibroblasts, promotes cellular infiltration and, importantly, leads to enhanced the accumulation of IL-17-producing gammadeltaT cells in skin, comprising a positive feed-forward mechanism. Thus, CIKS-mediated signaling is central in the development of both dermal and epidermal hallmarks of psoriasis, inducing distinct pathologies via target cell-specific effects. CIKS-mediated signaling represents a potential therapeutic target in psoriasis.
- Mus musculus (House mouse),
An AhR-Ovol1-Id1 regulatory axis in keratinocytes promotes skin homeostasis against atopic dermatitis
Preprint on BioRxiv : the Preprint Server for Biology on 31 January 2024 by Chen, Z., Dragan, M., et al.
PubMed
ABSTRACT Skin is our outer permeability and immune defense barrier against myriad external assaults. Aryl hydrocarbon receptor (AhR) senses environmental factors and regulates barrier robustness and immune homeostasis. AhR agonist is in clinical trial for atopic dermatitis (AD) treatment, but the underlying mechanism of action remains ill-defined. Here we report OVOL1/Ovol1 as a conserved and direct transcriptional target of AhR in epidermal keratinocytes. We show that OVOL1/Ovol1 impacts AhR regulation of keratinocyte gene expression, and Ovol1 deletion in keratinocytes hampers AhR’s barrier promotion function and worsens AD-like inflammation. Mechanistically, we identify Ovol1’s direct downstream targets genome-wide, and provide in vivo evidence for Id1’s critical role in barrier maintenance and disease suppression. Furthermore, our findings reveal an IL-1/dermal γδT cell axis exacerbating both type 2 and type 3 immune responses downstream of barrier perturbation in Ovol1 -deficient AD skin. Finally, we present data suggesting the clinical relevance of OVOL1 and ID1 function in human AD. Our study highlights a keratinocyte-intrinsic AhR-Ovol1-Id1 regulatory axis that promotes both epidermal and immune homeostasis against AD-like inflammation, implicating new therapeutic targets for AD.
Multiomic profiling of cutaneous leishmaniasis infections reveals microbiota-driven mechanisms underlying disease severity.
In Science Translational Medicine on 18 October 2023 by Amorim, C. F., Lovins, V. M., et al.
PubMed
Leishmania braziliensis is a parasitic infection that can result in inflammation and skin injury with highly variable and unpredictable clinical outcomes. Here, we investigated the potential impact of microbiota on infection-induced inflammatory responses and disease resolution by conducting an integrated analysis of the skin microbiome and host transcriptome on a cohort of 62 patients infected with L. braziliensis. We found that overall bacterial burden and microbiome configurations dominated with Staphylococcus spp. were associated with delayed healing and enhanced inflammatory responses, especially by IL-1 family members. Quantification of host and bacterial transcripts on human lesions revealed that high lesional S. aureus transcript abundance was associated with delayed healing and increased expression of IL-1β. This cytokine was critical for modulating disease outcomes in L. braziliensis-infected mice colonized with S. aureus, given that its neutralization reduced pathology and inflammation. These results highlight how the human microbiome can shape disease outcomes in cutaneous leishmaniasis and suggest pathways toward host-directed therapies to mitigate the inflammatory consequences.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
Early alveolar macrophage response and IL-1R-dependent T cell priming determine transmissibility of Mycobacterium tuberculosis strains.
In Nature Communications on 16 February 2022 by Lovey, A., Verma, S., et al.
PubMed
Mechanisms underlying variability in transmission of Mycobacterium tuberculosis strains remain undefined. By characterizing high and low transmission strains of M.tuberculosis in mice, we show here that high transmission M.tuberculosis strain induce rapid IL-1R-dependent alveolar macrophage migration from the alveolar space into the interstitium and that this action is key to subsequent temporal events of early dissemination of bacteria to the lymph nodes, Th1 priming, granulomatous response and bacterial control. In contrast, IL-1R-dependent alveolar macrophage migration and early dissemination of bacteria to lymph nodes is significantly impeded in infection with low transmission M.tuberculosis strain; these events promote the development of Th17 immunity, fostering neutrophilic inflammation and increased bacterial replication. Our results suggest that by inducing granulomas with the potential to develop into cavitary lesions that aids bacterial escape into the airways, high transmission M.tuberculosis strain is poised for greater transmissibility. These findings implicate bacterial heterogeneity as an important modifier of TB disease manifestations and transmission. © 2022. The Author(s).
- Cardiovascular biology,
- Immunology and Microbiology
Viral coinfection promotes tuberculosis immunopathogenesis by type I IFN signaling-dependent impediment of Th1 pulmonary influx
Preprint on Research Square on 23 June 2021 by Shin, S. J., Kang, T. G., et al.
PubMed
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is often exacerbated upon coinfection, but the underlying immunological mechanisms remain unclear. Here, to elucidate these mechanisms, we used a Mtb and lymphocytic choriomeningitis virus coinfection model. Viral coinfection significantly suppressed Mtb-specific IFN-γ production, with elevated bacterial loads and hyperinflammation in the lungs. Type I IFN signaling blockade rescued the Mtb-specific IFN-γ response and ameliorated lung immunopathology. Single-cell sequencing, tissue immunofluorescence staining, and adoptive transfer experiments revealed that type I IFN signaling produced in response to viral infection inhibited CXCL9/10 production in myeloid cells, resulting in impaired pulmonary migration of Mtb-specific CD4 + T cells from lymph nodes. Thus, virus coinfection-induced type I IFN signaling prior to the pulmonary localization of Mtb-specific Th1 cells exacerbates TB immunopathogenesis by impeding the Mtb-specific Th1 cell influx. Our study highlights another novel negative role of viral coinfection and/or type I IFNs in delaying Mtb-specific Th1 responses in the lung.
- In Vitro,
- Neutralization,
- Mus musculus (House mouse),
- Immunology and Microbiology
Naïve CD8 T cell IFNγ responses to a vacuolar antigen are regulated by an inflammasome-independent NLRP3 pathway and Toxoplasma gondii ROP5.
In PLoS Pathogens on 1 August 2020 by Kongsomboonvech, A. K., Rodríguez, F., et al.
PubMed
Host resistance to Toxoplasma gondii relies on CD8 T cell IFNγ responses, which if modulated by the host or parasite could influence chronic infection and parasite transmission between hosts. Since host-parasite interactions that govern this response are not fully elucidated, we investigated requirements for eliciting naïve CD8 T cell IFNγ responses to a vacuolar resident antigen of T. gondii, TGD057. Naïve TGD057 antigen-specific CD8 T cells (T57) were isolated from transnuclear mice and responded to parasite-infected bone marrow-derived macrophages (BMDMs) in an antigen-dependent manner, first by producing IL-2 and then IFNγ. T57 IFNγ responses to TGD057 were independent of the parasite's protein export machinery ASP5 and MYR1. Instead, host immunity pathways downstream of the regulatory Immunity-Related GTPases (IRG), including partial dependence on Guanylate-Binding Proteins, are required. Multiple T. gondii ROP5 isoforms and allele types, including 'avirulent' ROP5A from clade A and D parasite strains, were able to suppress CD8 T cell IFNγ responses to parasite-infected BMDMs. Phenotypic variance between clades B, C, D, F, and A strains suggest T57 IFNγ differentiation occurs independently of parasite virulence or any known IRG-ROP5 interaction. Consistent with this, removal of ROP5 is not enough to elicit maximal CD8 T cell IFNγ production to parasite-infected cells. Instead, macrophage expression of the pathogen sensors, NLRP3 and to a large extent NLRP1, were absolute requirements. Other members of the conventional inflammasome cascade are only partially required, as revealed by decreased but not abrogated T57 IFNγ responses to parasite-infected ASC, caspase-1/11, and gasdermin D deficient cells. Moreover, IFNγ production was only partially reduced in the absence of IL-12, IL-18 or IL-1R signaling. In summary, T. gondii effectors and host machinery that modulate parasitophorous vacuolar membranes, as well as NLR-dependent but inflammasome-independent pathways, determine the full commitment of CD8 T cells IFNγ responses to a vacuolar antigen.
- In Vivo,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Systemic dysfunction and plasticity of the immune macroenvironment in cancer models.
In Nature Medicine on 1 July 2020 by Allen, B. M., Hiam, K. J., et al.
PubMed
Understanding of the factors governing immune responses in cancer remains incomplete, limiting patient benefit. In this study, we used mass cytometry to define the systemic immune landscape in response to tumor development across five tissues in eight mouse tumor models. Systemic immunity was dramatically altered across models and time, with consistent findings in the peripheral blood of patients with breast cancer. Changes in peripheral tissues differed from those in the tumor microenvironment. Mice with tumor-experienced immune systems mounted dampened responses to orthogonal challenges, including reduced T cell activation during viral or bacterial infection. Antigen-presenting cells (APCs) mounted weaker responses in this context, whereas promoting APC activation rescued T cell activity. Systemic immune changes were reversed with surgical tumor resection, and many were prevented by interleukin-1 or granulocyte colony-stimulating factor blockade, revealing remarkable plasticity in the systemic immune state. These results demonstrate that tumor development dynamically reshapes the composition and function of the immune macroenvironment.
- Immunology and Microbiology
Neonatal-derived IL-17 producing dermal γδ T cells are required to prevent spontaneous atopic dermatitis
Preprint on BioRxiv : the Preprint Server for Biology on 28 June 2019 by Spidale, N., Malhotra, N., et al.
PubMed
h4>ABSTRACT/h4> Atopic Dermatitis (AD) is a T cell-mediated chronic skin disease and is associated with altered skin barrier integrity. Infants with mutations in genes involved in tissue barrier fitness are predisposed towards inflammatory diseases, but most do not develop or sustain the diseases, suggesting that there exist regulatory immune mechanisms to repair tissues and/or prevent aberrant inflammation. The absence of one single murine dermal cell type, the innate neonatal-derived IL-17 producing γδ T (Tγδ17) cells, from birth resulted in spontaneous, highly penetrant AD with all the major hallmarks of human AD. In Tγδ17 cell-deficient mice, basal keratinocyte transcriptome was altered months in advance of AD induction. Fulminant disease is driven by skin commensal bacteria dysbiosis and highly expanded dermal αβ T clonotypes that produce the type three cytokines, IL-17 and IL-22. These results demonstrate that neonatal Tγδ17 cells are innate skin regulatory T cells. The bifurcation of type 3 cytokine producing skin T cells into the homeostatic, early innate and pathogen-sensing, late adaptive T cell compartments underpin healthy skin and accounts for the dual function of type 3 cytokines in skin maintenance and inflammation.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
Anti-commensal IgG Drives Intestinal Inflammation and Type 17 Immunity in Ulcerative Colitis.
In Immunity on 16 April 2019 by Castro-Dopico, T., Dennison, T. W., et al.
PubMed
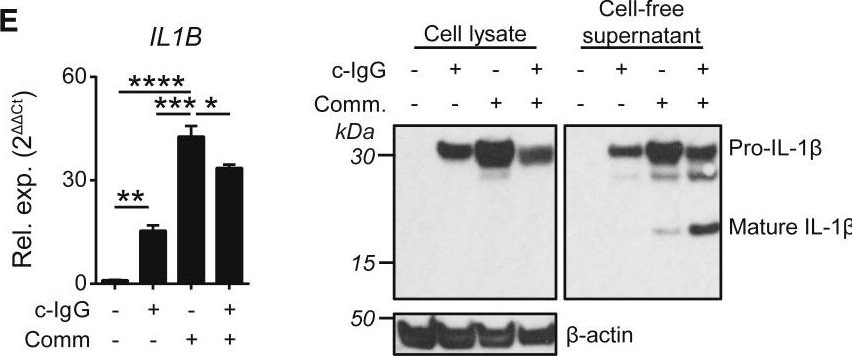
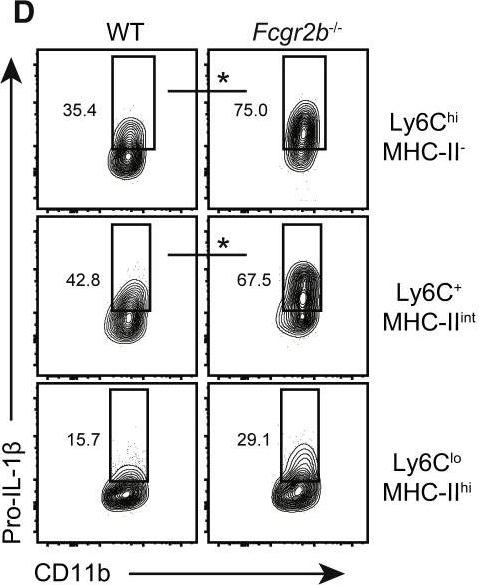
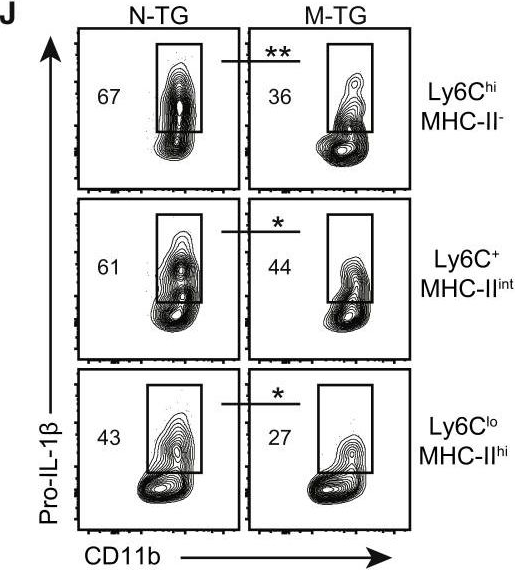
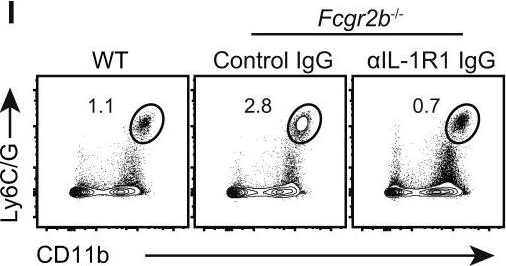
Inflammatory bowel disease is a chronic, relapsing condition with two subtypes, Crohn's disease (CD) and ulcerative colitis (UC). Genome-wide association studies (GWASs) in UC implicate a FCGR2A variant that alters the binding affinity of the antibody receptor it encodes, FcγRIIA, for immunoglobulin G (IgG). Here, we aimed to understand the mechanisms whereby changes in FcγRIIA affinity would affect inflammation in an IgA-dominated organ. We found a profound induction of anti-commensal IgG and a concomitant increase in activating FcγR signaling in the colonic mucosa of UC patients. Commensal-IgG immune complexes engaged gut-resident FcγR-expressing macrophages, inducing NLRP3- and reactive-oxygen-species-dependent production of interleukin-1β (IL-1β) and neutrophil-recruiting chemokines. These responses were modulated by the FCGR2A genotype. In vivo manipulation of macrophage FcγR signal strength in a mouse model of UC determined the magnitude of intestinal inflammation and IL-1β-dependent type 17 immunity. The identification of an important contribution of IgG-FcγR-dependent inflammation to UC has therapeutic implications.Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
- Inhib,
- Mus musculus (House mouse),
- Immunology and Microbiology
IL-6 receptor blockade corrects defects of XIAP-deficient regulatory T cells.
In Nature Communications on 31 January 2018 by Hsieh, W. C., Hsu, T. S., et al.
PubMed
X-linked lymphoproliferative syndrome type-2 (XLP-2) is a primary immunodeficiency disease attributed to XIAP mutation and is triggered by infection. Here, we show that mouse Xiap-/- regulatory T (Treg) cells and human XIAP-deficient Treg cells are defective in suppressive function. The Xiap-/- Treg cell defect is linked partly to decreased SOCS1 expression. XIAP binds SOCS1 and promotes SOCS1 stabilization. Foxp3 stability is reduced in Xiap-/- Treg cells. In addition, Xiap-/- Treg cells are prone to IFN-γ secretion. Transfer of wild-type Treg cells partly rescues infection-induced inflammation in Xiap-/- mice. Notably, inflammation-induced reprogramming of Xiap-/- Treg cells can be prevented by blockade of the IL-6 receptor (IL-6R), and a combination of anti-IL-6R and Xiap-/- Treg cells confers survival to inflammatory infection in Xiap-/- mice. Our results suggest that XLP-2 can be corrected by combination treatment with autologous iTreg (induced Treg) cells and anti-IL-6R antibody, bypassing the necessity to transduce Treg cells with XIAP.
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
Cutaneous Leishmaniasis Induces a Transmissible Dysbiotic Skin Microbiota that Promotes Skin Inflammation.
In Cell Host & Microbe on 12 July 2017 by Gimblet, C., Meisel, J. S., et al.
PubMed
Skin microbiota can impact allergic and autoimmune responses, wound healing, and anti-microbial defense. We investigated the role of skin microbiota in cutaneous leishmaniasis and found that human patients infected with Leishmania braziliensis develop dysbiotic skin microbiota, characterized by increases in the abundance of Staphylococcus and/or Streptococcus. Mice infected with L. major exhibit similar changes depending upon disease severity. Importantly, this dysbiosis is not limited to the lesion site, but is transmissible to normal skin distant from the infection site and to skin from co-housed naive mice. This observation allowed us to test whether a pre-existing dysbiotic skin microbiota influences disease, and we found that challenging dysbiotic naive mice with L. major or testing for contact hypersensitivity results in exacerbated skin inflammatory responses. These findings demonstrate that a dysbiotic skin microbiota is not only a consequence of tissue stress, but also enhances inflammation, which has implications for many inflammatory cutaneous diseases.Copyright © 2017 Elsevier Inc. All rights reserved.
- In Vitro,
- In Vivo,
- Mus musculus (House mouse),
- Immunology and Microbiology
IL-1-induced Bhlhe40 identifies pathogenic T helper cells in a model of autoimmune neuroinflammation.
In The Journal of Experimental Medicine on 8 February 2016 by Lin, C. C., Bradstreet, T. R., et al.
PubMed
The features that define autoreactive T helper (Th) cell pathogenicity remain obscure. We have previously shown that Th cells require the transcription factor Bhlhe40 to mediate experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. Here, using Bhlhe40 reporter mice and analyzing both polyclonal and TCR transgenic Th cells, we found that Bhlhe40 expression was heterogeneous after EAE induction, with Bhlhe40-expressing cells displaying marked production of IFN-γ, IL-17A, and granulocyte-macrophage colony-stimulating factor. In adoptive transfer EAE models, Bhlhe40-deficient Th1 and Th17 cells were both nonencephalitogenic. Pertussis toxin (PTX), a classical co-adjuvant for actively induced EAE, promoted IL-1β production by myeloid cells in the draining lymph node and served as a strong stimulus for Bhlhe40 expression in Th cells. Furthermore, PTX co-adjuvanticity was Bhlhe40 dependent. IL-1β induced Bhlhe40 expression in polarized Th17 cells, and Bhlhe40-expressing cells exhibited an encephalitogenic transcriptional signature. In vivo, IL-1R signaling was required for full Bhlhe40 expression by Th cells after immunization. Overall, we demonstrate that Bhlhe40 expression identifies encephalitogenic Th cells and defines a PTX-IL-1-Bhlhe40 pathway active in EAE. © 2016 Lin et al.
CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells.
In Nature Communications on 29 October 2015 by Kara, E. E., McKenzie, D. R., et al.
PubMed
IL-17-producing helper T (Th17) cells are critical for host defense against extracellular pathogens but also drive numerous autoimmune diseases. Th17 cells that differ in their inflammatory potential have been described including IL-10-producing Th17 cells that are weak inducers of inflammation and highly inflammatory, IL-23-driven, GM-CSF/IFNγ-producing Th17 cells. However, their distinct developmental requirements, functions and trafficking mechanisms in vivo remain poorly understood. Here we identify a temporally regulated IL-23-dependent switch from CCR6 to CCR2 usage by developing Th17 cells that is critical for pathogenic Th17 cell-driven inflammation in experimental autoimmune encephalomyelitis (EAE). This switch defines a unique in vivo cell surface signature (CCR6(-)CCR2(+)) of GM-CSF/IFNγ-producing Th17 cells in EAE and experimental persistent extracellular bacterial infection, and in humans. Using this signature, we identify an IL-23/IL-1/IFNγ/TNFα/T-bet/Eomesodermin-driven circuit driving GM-CSF/IFNγ-producing Th17 cell formation in vivo. Thus, our data identify a unique cell surface signature, trafficking mechanism and T-cell intrinsic regulators of GM-CSF/IFNγ-producing Th17 cells.
- In Vitro,
- Mus musculus (House mouse),
- Immunology and Microbiology
IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms.
In Proceedings of the National Academy of Sciences of the United States of America on 19 August 2014 by Ha, H. L., Wang, H., et al.
PubMed
Psoriasis is a chronic inflammatory skin disease characterized by abnormal keratinocyte proliferation and differentiation and by an influx of inflammatory cells. The mechanisms underlying psoriasis in humans and in mouse models are poorly understood, although evidence strongly points to crucial contributions of IL-17 cytokines, which signal via the obligatory adaptor CIKS/Act1. Here we identify critical roles of CIKS/Act1-mediated signaling in imiquimod-induced psoriatic inflammation, a mouse model that shares features with the human disease. We found that IL-17 cytokines/CIKS-mediated signaling into keratinocytes is essential for neutrophilic microabscess formation and contributes to hyperproliferation and markedly attenuated differentiation of keratinocytes, at least in part via direct effects. In contrast, IL-17 cytokines/CIKS-mediated signaling into nonkeratinocytes, particularly into dermal fibroblasts, promotes cellular infiltration and, importantly, leads to enhanced the accumulation of IL-17-producing γδT cells in skin, comprising a positive feed-forward mechanism. Thus, CIKS-mediated signaling is central in the development of both dermal and epidermal hallmarks of psoriasis, inducing distinct pathologies via target cell-specific effects. CIKS-mediated signaling represents a potential therapeutic target in psoriasis.