InVivoMAb anti-mouse MHC Class I (H-2)
Product Details
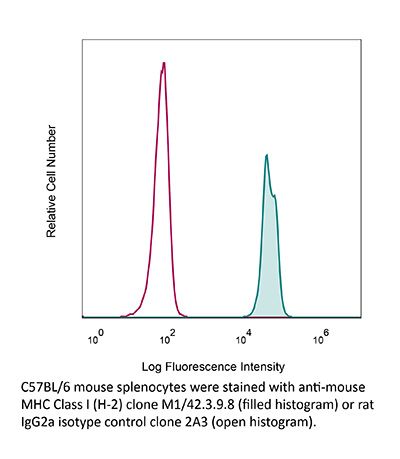
The M1/42.3.9.8 monoclonal antibody reacts with the mouse H-2 MHC class I alloantigen (all haplotypes). MHC class I antigens are heterodimers consisting of one alpha chain (44 kDa) associated with ß2 microglobulin (11.5 kDa). The antigen is expressed by all nucleated cells at varying levels. MHC Class I molecules present endogenously synthesized antigenic peptides to CD8 T cells.Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | C57BL/10 mouse spleen cells enriched for T cells |
| Reported Applications |
ex vivo blocking of MHC I-dependent interactions Immunofluorescence Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1125537 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
ex vivo blocking of MHC I–dependent interactions
Herz, J., et al. (2015). "Therapeutic antiviral T cells noncytopathically clear persistently infected microglia after conversion into antigen-presenting cells" J Exp Med 212(8): 1153-1169. PubMed
Several viruses can infect the mammalian nervous system and induce neurological dysfunction. Adoptive immunotherapy is an approach that involves administration of antiviral T cells and has shown promise in clinical studies for the treatment of peripheral virus infections in humans such as cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus, among others. In contrast, clearance of neurotropic infections is particularly challenging because the central nervous system (CNS) is relatively intolerant of immunopathological reactions. Therefore, it is essential to develop and mechanistically understand therapies that noncytopathically eradicate pathogens from the CNS. Here, we used mice persistently infected from birth with lymphocytic choriomeningitis virus (LCMV) to demonstrate that therapeutic antiviral T cells can completely purge the persistently infected brain without causing blood-brain barrier breakdown or tissue damage. Mechanistically, this is accomplished through a tailored release of chemoattractants that recruit antiviral T cells, but few pathogenic innate immune cells such as neutrophils and inflammatory monocytes. Upon arrival, T cells enlisted the support of nearly all brain-resident myeloid cells (microglia) by inducing proliferation and converting them into CD11c(+) antigen-presenting cells (APCs). Two-photon imaging experiments revealed that antiviral CD8(+) and CD4(+) T cells interacted directly with CD11c(+) microglia and induced STAT1 signaling but did not initiate programmed cell death. We propose that noncytopathic CNS viral clearance can be achieved by therapeutic antiviral T cells reliant on restricted chemoattractant production and interactions with apoptosis-resistant microglia.
Immunofluorescence
Rockett, B. D., et al. (2011). "Membrane raft organization is more sensitive to disruption by (n-3) PUFA than nonraft organization in EL4 and B cells" J Nutr 141(6): 1041-1048. PubMed
Model membrane and cellular detergent extraction studies show (n-3) PUFA predominately incorporate into nonrafts; thus, we hypothesized (n-3) PUFA could disrupt nonraft organization. The first objective of this study was to determine whether (n-3) PUFA disrupted nonrafts of EL4 cells, an extension of our previous work in which we discovered an (n-3) PUFA diminished raft clustering. EPA or DHA treatment of EL4 cells increased plasma membrane accumulation of the nonraft probe 1,1′-dilinoleyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate by ~50-70% relative to a BSA control. Forster resonance energy transfer imaging showed EPA and DHA also disrupted EL4 nanometer scale nonraft organization by increasing the distance between nonraft molecules by ~25% compared with BSA. However, changes in nonrafts were due to an increase in cell size; under conditions where EPA or DHA did not increase cell size, nonraft organization was unaffected. We next translated findings on EL4 cells by testing if (n-3) PUFA administered to mice disrupted nonrafts and rafts. Imaging of B cells isolated from mice fed low- or high-fat (HF) (n-3) PUFA diets showed no change in nonraft organization compared with a control diet (CD). However, confocal microscopy revealed the HF (n-3) PUFA diet disrupted lipid raft clustering and size by ~40% relative to CD. Taken together, our data from 2 different model systems suggest (n-3) PUFA have limited effects on nonrafts. The ex vivo data, which confirm previous studies with EL4 cells, provide evidence that (n-3) PUFA consumed through the diet disrupt B cell lipid raft clustering.
Flow Cytometry, Immunofluorescence
Shaikh, S. R., et al. (2009). "Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells" J Nutr 139(9): 1632-1639. PubMed
An emerging molecular mechanism by which docosahexaenoic acid (DHA) exerts its effects is modification of lipid raft organization. The biophysical model, based on studies with liposomes, shows that DHA avoids lipid rafts because of steric incompatibility between DHA and cholesterol. The model predicts that DHA does not directly modify rafts; rather, it incorporates into nonrafts to modify the lateral organization and/or conformation of membrane proteins, such as the major histocompatibility complex (MHC) class I. Here, we tested predictions of the model at a cellular level by incorporating oleic acid, eicosapentaenoic acid (EPA), and DHA, compared with a bovine serum albumin (BSA) control, into the membranes of EL4 cells. Quantitative microscopy showed that DHA, but not EPA, treatment, relative to the BSA control diminished lipid raft clustering and increased their size. Approximately 30% of DHA was incorporated directly into rafts without changing the distribution of cholesterol between rafts and nonrafts. Quantification of fluorescence colocalization images showed that DHA selectively altered MHC class I lateral organization by increasing the fraction of the nonraft protein into rafts compared with BSA. Both DHA and EPA treatments increased antibody binding to MHC class I compared with BSA. Antibody titration showed that DHA and EPA did not change MHC I conformation but increased total surface levels relative to BSA. Taken together, our findings are not in agreement with the biophysical model. Therefore, we propose a model that reconciles contradictory viewpoints from biophysical and cellular studies to explain how DHA modifies lipid rafts on several length scales. Our study supports the notion that rafts are an important target of DHA’s mode of action.
- Mus musculus (House mouse),
Immunopeptidome mining reveals a novel ERS-induced target in T1D
Preprint on Research Square on 9 June 2023 by Wang, L., Li, J., et al.
PubMed
Autoreactive CD8 + T cells play a key role in type 1 diabetes (T1D), but the antigen spectrum that activates autoreactive CD8 + T cells remains unclear. Endoplasmic reticulum stress (ERS) has been implicated in β cell autoantigen generation. Here, we analyzed the major histocompatibility complex class I (MHC-I)-associated immunopeptidome (MIP) of islet β cells under steady-state and ERS conditions and found a small number of peptides that were exclusively present in the MIP of the ERS-exposed β cell line. Among them, OTUB2 58 − 66 showed immunodominance, and the corresponding autoreactive CD8 + T cells were diabetogenic in NOD mice. High glucose intake upregulated pancreatic OTUB2 expression and amplified the OTUB2 58 − 66 -specific CD8 + T-cell response in NOD mice. Repeated OTUB2 58 − 66 administration significantly reduced the T1D incidence in these mice. This study provides novel β cell autoantigens for developing specific immune interventions for T1D prevention and treatment. Data are available via ProteomeXchange with identifier PXD041227.
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Cellular Senescence Is Immunogenic and Promotes Antitumor Immunity.
In Cancer Discovery on 6 February 2023 by Marin, I., Boix, O., et al.
PubMed
Cellular senescence is a stress response that activates innate immune cells, but little is known about its interplay with the adaptive immune system. Here, we show that senescent cells combine several features that render them highly efficient in activating dendritic cells (DC) and antigen-specific CD8 T cells. This includes the release of alarmins, activation of IFN signaling, enhanced MHC class I machinery, and presentation of senescence-associated self-peptides that can activate CD8 T cells. In the context of cancer, immunization with senescent cancer cells elicits strong antitumor protection mediated by DCs and CD8 T cells. Interestingly, this protection is superior to immunization with cancer cells undergoing immunogenic cell death. Finally, the induction of senescence in human primary cancer cells also augments their ability to activate autologous antigen-specific tumor-infiltrating CD8 lymphocytes. Our study indicates that senescent cancer cells can be exploited to develop efficient and protective CD8-dependent antitumor immune responses. Our study shows that senescent cells are endowed with a high immunogenic potential-superior to the gold standard of immunogenic cell death. We harness these properties of senescent cells to trigger efficient and protective CD8-dependent antitumor immune responses. See related article by Chen et al., p. 432. This article is highlighted in the In This Issue feature, p. 247. ©2022 The Authors; Published by the American Association for Cancer Research.
- Mus musculus (House mouse),
- Cancer Research
Induction of senescence renders cancer cells highly immunogenic
Preprint on BioRxiv : the Preprint Server for Biology on 6 June 2022 by Marín, I., Boix, O., et al.
PubMed
h4>ABSTRACT/h4> Cellular senescence is a stress response that activates innate immunity. However, the interplay between senescent cells and the adaptive immune system remains largely unexplored. Here, we show that senescent cells display enhanced MHC class I (MHC-I) antigen processing and presentation. Immunization of mice with senescent syngeneic fibroblasts generates CD8 T cells reactive against both normal and senescent fibroblasts, some of them targeting senescence-associated MHC-I-peptides. In the context of cancer, we demonstrate that senescent cancer cells trigger strong anti-tumor protection mediated by antigen-presenting cells and CD8 T cells. This response is superior to the protection elicited by cells undergoing immunogenic cell death. Finally, induction of senescence in patient-derived cancer cells exacerbates the activation of autologous tumor-reactive CD8 tumor-infiltrating lymphocytes (TILs) with no effect on non-reactive TILs. Our study indicates that immunization with senescent cancer cells strongly activates anti-tumor immunity, and this can be exploited for cancer therapy. h4>STATEMENT OF SIGNIFICANCE/h4> Our study shows that senescent cells are endowed with a high immunogenic potential, superior to the gold standard of immunogenic cell death. The induction of senescence in cancer cells can be exploited to develop efficient and protective CD8-dependent anti-tumor immune responses.
- In Vivo,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Adoptive immunotherapy with transient anti-CD4 treatment enhances anti-tumor response by increasing IL-18Rαhi CD8+ T cells.
In Nature Communications on 7 September 2021 by Kim, S. H., Cho, E., et al.
PubMed
Adoptive T cell therapy (ACT) requires lymphodepletion preconditioning to eliminate immune-suppressive elements and enable efficient engraftment of adoptively transferred tumor-reactive T cells. As anti-CD4 monoclonal antibody depletes CD4+ immune-suppressive cells, the combination of anti-CD4 treatment and ACT has synergistic potential in cancer therapy. Here, we demonstrate a post-ACT conditioning regimen that involves transient anti-CD4 treatment (CD4post). Using murine melanoma, the combined effect of cyclophosphamide preconditioning (CTXpre), CD4post, and ex vivo primed tumor-reactive CD8+ T-cell infusion is presented. CTXpre/CD4post increases tumor suppression and host survival by accelerating the proliferation and differentiation of ex vivo primed CD8+ T cells and endogenous CD8+ T cells. Endogenous CD8+ T cells enhance effector profile and tumor-reactivity, indicating skewing of the TCR repertoire. Notably, enrichment of polyfunctional IL-18Rαhi CD8+ T cell subset is the key event in CTXpre/CD4post-induced tumor suppression. Mechanistically, the anti-tumor effect of IL-18Rαhi subset is mediated by IL-18 signaling and TCR-MHC I interaction. This study highlights the clinical relevance of CD4post in ACT and provides insights regarding the immunological nature of anti-CD4 treatment, which enhances anti-tumor response of CD8+ T cells. © 2021. The Author(s).
- In Vitro,
- Mus musculus (House mouse),
- Immunology and Microbiology,
- Pathology
Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH.
In Nature on 1 April 2021 by Dudek, M., Pfister, D., et al.
PubMed
Nonalcoholic steatohepatitis (NASH) is a manifestation of systemic metabolic disease related to obesity, and causes liver disease and cancer1,2. The accumulation of metabolites leads to cell stress and inflammation in the liver3, but mechanistic understandings of liver damage in NASH are incomplete. Here, using a preclinical mouse model that displays key features of human NASH (hereafter, NASH mice), we found an indispensable role for T cells in liver immunopathology. We detected the hepatic accumulation of CD8 T cells with phenotypes that combined tissue residency (CXCR6) with effector (granzyme) and exhaustion (PD1) characteristics. Liver CXCR6+ CD8 T cells were characterized by low activity of the FOXO1 transcription factor, and were abundant in NASH mice and in patients with NASH. Mechanistically, IL-15 induced FOXO1 downregulation and CXCR6 upregulation, which together rendered liver-resident CXCR6+ CD8 T cells susceptible to metabolic stimuli (including acetate and extracellular ATP) and collectively triggered auto-aggression. CXCR6+ CD8 T cells from the livers of NASH mice or of patients with NASH had similar transcriptional signatures, and showed auto-aggressive killing of cells in an MHC-class-I-independent fashion after signalling through P2X7 purinergic receptors. This killing by auto-aggressive CD8 T cells fundamentally differed from that by antigen-specific cells, which mechanistically distinguishes auto-aggressive and protective T cell immunity.
- Block,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control.
In The Journal of Clinical Investigation on 1 March 2021 by Lhuillier, C., Rudqvist, N. P., et al.
PubMed
Neoantigens generated by somatic nonsynonymous mutations are key targets of tumor-specific T cells, but only a small number of mutations predicted to be immunogenic are presented by MHC molecules on cancer cells. Vaccination studies in mice and patients have shown that the majority of neoepitopes that elicit T cell responses fail to induce significant antitumor activity, for incompletely understood reasons. We report that radiotherapy upregulates the expression of genes containing immunogenic mutations in a poorly immunogenic mouse model of triple-negative breast cancer. Vaccination with neoepitopes encoded by these genes elicited CD8+ and CD4+ T cells that, whereas ineffective in preventing tumor growth, improved the therapeutic efficacy of radiotherapy. Mechanistically, neoantigen-specific CD8+ T cells preferentially killed irradiated tumor cells. Neoantigen-specific CD4+ T cells were required for the therapeutic efficacy of vaccination and acted by producing Th1 cytokines, killing irradiated tumor cells, and promoting epitope spread. Such a cytotoxic activity relied on the ability of radiation to upregulate class II MHC molecules as well as the death receptors FAS/CD95 and DR5 on the surface of tumor cells. These results provide proof-of-principle evidence that radiotherapy works in concert with neoantigen vaccination to improve tumor control.
- Immu-puri,
- Mus musculus (House mouse),
- Immunology and Microbiology
An input-controlled model system for identification of MHC bound peptides enabling laboratory comparisons of immunopeptidome experiments.
In Journal of Proteomics on 30 September 2020 by Klatt, M. G., Aretz, Z. E. H., et al.
PubMed
Characterization of MHC-bound peptides by mass spectrometry (MS) is an essential technique for immunologic studies. Many efforts have been made to quantify the number of MHC-presented ligands by MS and to define the limits of detection of a specific MHC ligand. However, these experiments are often complex and comparisons across different laboratories are challenging. Therefore, we compared and orthogonally validated quantitation of peptide:MHC complexes by radioimmunoassay and flow cytometry using TCR mimic antibodies in three model systems to establish a method to control the experimental input of peptide MHC:complexes for MS analysis. Following isolation of MHC-bound peptides we identified and quantified an MHC ligand of interest with high correlation to the initial input. We found that the diversity of the presented ligandome, as well as the peptide sequence itself affected the detection of the target peptide. Furthermore, results were applicable from these model systems to unmodified cell lines with a tight correlation between HLA-A*02 complex input and the number of identified HLA-A*02 ligands. Overall, this framework provides an easily accessible experimental setup that offers the opportunity to control the peptide:MHC input and in this way compare immunopeptidome experiments not only within but also between laboratories, independent of their experimental approach. SIGNIFICANCE: Although immunopeptidomics is an essential tool for the characterization of MHCbound peptides on the cell surface, there are no easily applicable established protocols available that allow comparison of immunopeptidome experiments across laboratories. Here, we demonstrate that controlling the peptide:MHC input for immunopurification and LC-MS/MS experiments by flow cytometry in pre-defined model systems allows the generation of qualitative and quantitative data that can easily be compared between investigators, independently of their methods for MHC ligand isolation for MS. Copyright © 2020 Elsevier B.V. All rights reserved.
- Block,
- Mus musculus (House mouse),
- Immunology and Microbiology,
- Neuroscience
Eomes identifies thymic precursors of self-specific memory-phenotype CD8+ T cells.
In Nature Immunology on 1 May 2020 by Miller, C. H., Klawon, D. E. J., et al.
PubMed
Unprimed mice harbor a substantial population of 'memory-phenotype' CD8+ T cells (CD8-MP cells) that exhibit hallmarks of activation and innate-like functional properties. Due to the lack of faithful markers to distinguish CD8-MP cells from bona fide CD8+ memory T cells, the developmental origins and antigen specificities of CD8-MP cells remain incompletely defined. Using deep T cell antigen receptor (TCR) sequencing, we found that the TCRs expressed by CD8-MP cells are highly recurrent and distinct from the TCRs expressed by naive-phenotype CD8+ T cells. CD8-MP clones exhibited reactivity to widely expressed self-ligands. T cell precursors expressing CD8-MP TCRs showed upregulation of the transcription factor Eomes during maturation in the thymus, prior to induction of the full memory phenotype, which is suggestive of a unique program triggered by recognition of self-ligands. Moreover, CD8-MP cells infiltrate oncogene-driven prostate tumors and express high densities of PD-1, which suggests potential roles in antitumor immunity and the response to immunotherapy.
- Functional,
- Mus musculus (House mouse),
- Cancer Research,
- Immunology and Microbiology
ASTX660, an antagonist of cIAP1/2 and XIAP, increases antigen processing machinery and can enhance radiation-induced immunogenic cell death in preclinical models of head and neck cancer.
In Oncoimmunology on 1 February 2020 by Ye, W., Gunti, S., et al.
PubMed
Inhibitor of apoptosis protein (IAP) antagonists have shown activity in preclinical models of head and neck squamous cell carcinoma (HNSCC), and work across several cancer types has demonstrated diverse immune stimulatory effects including enhancement of T cell, NK cell, and dendritic cell function. However, tumor-cell-intrinsic mechanisms for this immune upregulation have been largely unexplored. In this study, we show that ASTX660, an antagonist of cIAP1/2 and XIAP, induces expression of immunogenic cell death (ICD) markers in sensitive HNSCC cell lines in vitro. Experiments in syngeneic mouse models of HNSCC showed that ASTX660 can also enhance radiation-induced ICD in vivo. On a functional level, ASTX660 also enhanced killing of multiple murine cell lines by cytotoxic tumor-infiltrating lymphocytes, and when combined with XRT, stimulated clonal expansion of antigen-specific T lymphocytes and expression of MHC class I on the surface of tumor cells. Flow cytometry experiments in several human HNSCC cell lines showed that MHC class I (HLA-A,B,C) was reliably upregulated in response to ASTX660 + TNFα, while increases in other antigen processing machinery (APM) components were variable among different cell lines. These findings suggest that ASTX660 may enhance anti-tumor immunity both by promoting ICD and by enhancing antigen processing and presentation. © 2020 The Author(s). Published with license by Taylor Francis Group, LLC.