InVivoMAb anti-mouse CD80 (B7-1)
Product Details
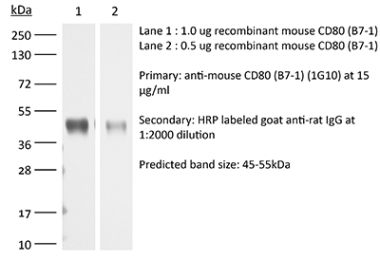
The 16-10A1 monoclonal antibody reacts with mouse CD80 also known as B7-1. CD80 is a 60 kDa Ig superfamily member and is expressed by activated B cells and constitutively by monocytes and dendritic cells. This ligand binds to CD28 to provide a costimulatory signal necessary for T cell activation and survival, and cytokine production. Additionally, CD80 binds to CTLA-4 which inhibits T cells. This antibody has been shown to block CD80 in vivo.Specifications
| Isotype | Armenian Hamster IgG2 |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb polyclonal Armenian hamster IgG |
| Recommended Dilution Buffer | InVivoPure pH 8.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | CHO cell line transfected with mouse CD80 |
| Reported Applications |
in vivo CD80 blockade Flow cytometry |
| Formulation |
PBS, pH 8.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein A |
| RRID | AB_1107676 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Recommended Products
in vivo CD80 blockade
Marshall, D., et al. (2014). "Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells" J Immunol 193(11): 5525-5533. PubMed
The developmental pathways of regulatory T cells (T(reg)) generation in the thymus are not fully understood. In this study, we reconstituted thymic development of Zap70-deficient thymocytes with a tetracycline-inducible Zap70 transgene to allow temporal dissection of T(reg) development. We find that T(reg) develop with distinctive kinetics, first appearing by day 4 among CD4 single-positive (SP) thymocytes. Accepted models of CD25(+)Foxp3(+) T(reg) selection suggest development via CD25(+)Foxp3(-) CD4 SP precursors. In contrast, our kinetic analysis revealed the presence of abundant CD25(-)Foxp3(+) cells that are highly efficient at maturing to CD25(+)Foxp3(+) cells in response to IL-2. CD25(-)Foxp3(+) cells more closely resembled mature T(reg) both with respect to kinetics of development and avidity for self-peptide MHC. These population also exhibited distinct requirements for cytokines during their development. CD25(-)Foxp3(+) cells were IL-15 dependent, whereas generation of CD25(+)Foxp3(+) specifically required IL-2. Finally, we found that IL-2 and IL-15 arose from distinct sources in vivo. IL-15 was of stromal origin, whereas IL-2 was of exclusively from hemopoetic cells that depended on intact CD4 lineage development but not either Ag-experienced or NKT cells.
in vivo CD80 blockade, Flow Cytometry
Moser, E. K., et al. (2014). "Late engagement of CD86 after influenza virus clearance promotes recovery in a FoxP3+ regulatory T cell dependent manner" PLoS Pathog 10(8): e1004315. PubMed
Influenza A virus (IAV) infection in the respiratory tract triggers robust innate and adaptive immune responses, resulting in both virus clearance and lung inflammation and injury. After virus clearance, resolution of ongoing inflammation and tissue repair occur during a distinct recovery period. B7 family co-stimulatory molecules such as CD80 and CD86 have important roles in modulating T cell activity during the initiation and effector stages of the host response to IAV infection, but their potential role during recovery and resolution of inflammation is unknown. We found that antibody-mediated CD86 blockade in vivo after virus clearance led to a delay in recovery, characterized by increased numbers of lung neutrophils and inflammatory cytokines in airways and lung interstitium, but no change in conventional IAV-specific T cell responses. However, CD86 blockade led to decreased numbers of FoxP3+ regulatory T cells (Tregs), and adoptive transfer of Tregs into alphaCD86 treated mice rescued the effect of the blockade, supporting a role for Tregs in promoting recovery after virus clearance. Specific depletion of Tregs late after infection mimicked the CD86 blockade phenotype, confirming a role for Tregs during recovery after virus clearance. Furthermore, we identified neutrophils as a target of Treg suppression since neutrophil depletion in Treg-depleted mice reduced excess inflammatory cytokines in the airways. These results demonstrate that Tregs, in a CD86 dependent mechanism, contribute to the resolution of disease after IAV infection, in part by suppressing neutrophil-driven cytokine release into the airways.
in vivo CD80 blockade
Srivastava, S., et al. (2014). "Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection" J Exp Med 211(5): 961-974. PubMed
Regulatory T (T reg) cells play an essential role in preventing autoimmunity but can also impair clearance of foreign pathogens. Paradoxically, signals known to promote T reg cell function are abundant during infection and could inappropriately enhance T reg cell activity. How T reg cell function is restrained during infection to allow the generation of effective antiviral responses remains largely unclear. We demonstrate that the potent antiviral type I interferons (IFNs) directly inhibit co-stimulation-dependent T reg cell activation and proliferation, both in vitro and in vivo during acute infection with lymphocytic choriomeningitis virus (LCMV). Loss of the type I IFN receptor specifically in T reg cells results in functional impairment of virus-specific CD8(+) and CD4(+) T cells and inefficient viral clearance. Together, these data demonstrate that inhibition of T reg cells by IFNs is necessary for the generation of optimal antiviral T cell responses during acute LCMV infection.
in vivo CD80 blockade
Arjunaraja, S., et al. (2012). "Structurally identical capsular polysaccharide expressed by intact group B streptococcus versus Streptococcus pneumoniae elicits distinct murine polysaccharide-specific IgG responses in vivo" J Immunol 188(11): 5238-5246. PubMed
We previously reported distinct differences in the murine in vivo Ig polysaccharide (PS)-specific responses to intact Streptococcus pneumoniae compared with responses to Neisseria meningitidis and that in each case, the bacterial subcapsular domain markedly influences the Ig response to the associated PS. In light of potentially unique contributions of biochemically distinct capsular PS and/or their characteristic attachments to the underlying bacterium, it remains unresolved whether different bacterial subcapsular domains can exert differential effects on PS-specific Ig responses to distinct bacterial pathogens. In this report, we used a mutant strain of group B Streptococcus (Streptococcus agalactiae) type III (GBS-III) that expresses desialylated capsular polysaccharide of GBS-III, biochemically identical to capsular pneumococcal polysaccharide type 14 (PPS14) of Streptococcus pneumoniae (intact inactivated Streptococcus pneumoniae, capsular type 14, Pn14), directly to compare the in vivo PPS14-specific IgG responses to two distinct gram-positive bacteria. Although both GBS-III and Pn14 elicited relatively rapid primary PPS14-specific IgG responses dependent on CD4(+) T cells, B7-dependent costimulation, and CD40-CD40L interactions, only GBS-III induced a highly boosted ICOS-dependent PPS14-specific IgG response after secondary immunization. Of note, priming with Pn14 and boosting with GBS-III, although not isolated PPS14, elicited a similar boosted PPS14-specific IgG response that was dependent on CD4(+) T cells during secondary immunization, indicating that Pn14 primes for memory but, unlike GBS-III, fails to elicit it. The inability of Pn14 to elicit a boosted PPS14-specific IgG response was overcome by coimmunization with unencapsulated GBS-III. Collectively, these data establish that structurally identical capsular PS expressed by two distinct gram-positive extracellular bacteria can indeed elicit distinct PS-specific IgG responses in vivo.
Flow Cytometry
Rockett, B. D., et al. (2010). "n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo" J Lipid Res 51(6): 1284-1297. PubMed
n-3 polyunsaturated fatty acids (PUFAs) modify T-cell activation, in part by remodeling lipid composition; however, the relationship between n-3 PUFA and B-cell activation is unknown. Here we tested this relationship in vitro and ex vivo by measuring upregulation of B-cell surface molecules, the percentage of cells activated, and cytokine secreted in response to lipopolysaccharide (LPS) activation. In vitro, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) improved the membrane n-6/n-3 PUFA ratio, and DHA lowered interleukin (IL)-6 secretion; overall, n-3 PUFAs did not suppress B-cell activation compared with BSA, oleate, or elaidate treatment. Palmitate treatment suppressed the percentage of B cells activated through lipoapoptosis, which was differentially prevented by cosupplementing cells with MUFAs and PUFAs. Ex vivo, we tested the hypothesis with mice fed a control or high-fat saturated, hydrogenated, MUFA or n-3 PUFA diets. n-3 PUFAs had no effect on the percentage of B cells activated. Unexpectedly, the n-3 PUFA diet increased B-cell CD69 surface expression, IL-6 and IFNgamma secretion, and it significantly increased body weight gain. Overall, we propose that changes in lipid composition with n-3 PUFA and suppression of lymphocyte activation is not universal. The study highlights that high-fat n-3 PUFA diets can promote pro-inflammatory responses, at least from one cell type.
- Cancer Research,
- Mus musculus (House mouse)
EMT activates exocytotic Rabs to coordinate invasion and immunosuppression in lung cancer.
In Proceedings of the National Academy of Sciences of the United States of America on 11 July 2023 by Xiao, G. Y., Tan, X., et al.
PubMed
Epithelial-to-mesenchymal transition (EMT) underlies immunosuppression, drug resistance, and metastasis in epithelial malignancies. However, the way in which EMT orchestrates disparate biological processes remains unclear. Here, we identify an EMT-activated vesicular trafficking network that coordinates promigratory focal adhesion dynamics with an immunosuppressive secretory program in lung adenocarcinoma (LUAD). The EMT-activating transcription factor ZEB1 drives exocytotic vesicular trafficking by relieving Rab6A, Rab8A, and guanine nucleotide exchange factors from miR-148a-dependent silencing, thereby facilitating MMP14-dependent focal adhesion turnover in LUAD cells and autotaxin-mediated CD8+ T cell exhaustion, indicating that cell-intrinsic and extrinsic processes are linked through a microRNA that coordinates vesicular trafficking networks. Blockade of ZEB1-dependent secretion reactivates antitumor immunity and negates resistance to PD-L1 immune checkpoint blockade, an important clinical problem in LUAD. Thus, EMT activates exocytotic Rabs to drive a secretory program that promotes invasion and immunosuppression in LUAD.
- Mus musculus (House mouse),
- Cardiovascular biology,
- Immunology and Microbiology
Pulmonary Dendritic Cell Subsets Shape the Respiratory Syncytial Virus-Specific CD8+ T Cell Immunodominance Hierarchy in Neonates.
In The Journal of Immunology on 1 January 2017 by Malloy, A. M., Ruckwardt, T. J., et al.
PubMed
Young infants are generally more susceptible to viral infections and experience more severe disease than do adults. CD8+ T cells are important for viral clearance, and although often ineffective in neonates they can be protective when adequately stimulated. Using a murine CB6F1/J hybrid model of respiratory syncytial virus (RSV) infection, we previously demonstrated that the CD8+ T cell immunodominance hierarchy to two RSV-derived epitopes, KdM282-90 and DbM187-195, was determined by the age at infection. To determine whether age-dependent RSV-specific CD8+ T cell responses could be modified through enhanced innate signaling, we used TLR4 or TLR9 agonist treatment at the time of infection, which remarkably changed the neonatal codominant response to an adult-like KdM282-90 CD8+ T cell immunodominant response. This shift was associated with an increase in the number of conventional dendritic cells, CD11b+ and CD103+ dendritic cells, in the lung-draining lymph node, as well as increased expression of the costimulatory molecule CD86. The magnitude of the KdM282-90 CD8+ T cell response in TLR agonist-treated neonates could be blocked with Abs against CD80 and CD86. These studies demonstrate the age-dependent function of conventional dendritic cells, their role in determining immunodominance hierarchy, and epitope-specific CD8+ T cell requirements for costimulation, all of which influence the immune response magnitude. The unique impact of TLR agonists on neonatal T cell responses is important to consider for RSV vaccines designed for young infants.
- Immunology and Microbiology
Acute Virus Control Mediated by Licensed NK Cells Sets Primary CD8+ T Cell Dependence on CD27 Costimulation.
In The Journal of Immunology on 1 December 2016 by Teoh, J. J., Gamache, A. E., et al.
PubMed
NK cells represent a critical first-line of immune defense against a bevy of viral pathogens, and infection can provoke them to mediate supportive and suppressive effects on virus-specific adaptive immunity. In mice expressing MHC class I Dk (Dk), a major murine CMV (MCMV) resistance factor and self-ligand of the inhibitory Ly49G2 (G2) receptor, licensed G2+ NK cells provide essential host resistance against MCMV infection. Additionally G2+ NK cell responses to MCMV increase the rate and extent of dendritic cell (DC) recovery, as well as early priming of CD8+ T cell effectors in response to MCMV. However, relatively little is known about the NK cell effect on costimulatory ligand patterns displayed by DCs or on ensuing effector and memory T cell responses. In this study, we found that CD27-dependent CD8+ T cell priming and differentiation are shaped by the efficiency of NK responses to virus infection. Surprisingly, differences in specific NK responses to MCMV in Dk-disparate mice failed to distinguish early DC costimulatory patterns. Nonetheless, although CD27 deficiency did not impede licensed NK-mediated resistance, CD70 and CD27 were required to efficiently prime and regulate effector CD8+ T cell differentiation in response to MCMV, which eventually resulted in biased memory T cell precursor formation in Dk mice. In contrast, CD8+ T cells accrued more slowly in non-Dk mice and eventually differentiated into terminal effector cells regardless of CD27 stimulation. Disparity in this requirement for CD27 signaling indicates that specific virus control mediated by NK cells can shape DC costimulatory signals needed to prime CD8+ T cells and eventual T cell fate decisions. Copyright © 2016 by The American Association of Immunologists, Inc.
- Immunology and Microbiology
Late engagement of CD86 after influenza virus clearance promotes recovery in a FoxP3+ regulatory T cell dependent manner.
In PLoS Pathogens on 1 August 2014 by Moser, E. K., Hufford, M. M., et al.
PubMed
Influenza A virus (IAV) infection in the respiratory tract triggers robust innate and adaptive immune responses, resulting in both virus clearance and lung inflammation and injury. After virus clearance, resolution of ongoing inflammation and tissue repair occur during a distinct recovery period. B7 family co-stimulatory molecules such as CD80 and CD86 have important roles in modulating T cell activity during the initiation and effector stages of the host response to IAV infection, but their potential role during recovery and resolution of inflammation is unknown. We found that antibody-mediated CD86 blockade in vivo after virus clearance led to a delay in recovery, characterized by increased numbers of lung neutrophils and inflammatory cytokines in airways and lung interstitium, but no change in conventional IAV-specific T cell responses. However, CD86 blockade led to decreased numbers of FoxP3+ regulatory T cells (Tregs), and adoptive transfer of Tregs into αCD86 treated mice rescued the effect of the blockade, supporting a role for Tregs in promoting recovery after virus clearance. Specific depletion of Tregs late after infection mimicked the CD86 blockade phenotype, confirming a role for Tregs during recovery after virus clearance. Furthermore, we identified neutrophils as a target of Treg suppression since neutrophil depletion in Treg-depleted mice reduced excess inflammatory cytokines in the airways. These results demonstrate that Tregs, in a CD86 dependent mechanism, contribute to the resolution of disease after IAV infection, in part by suppressing neutrophil-driven cytokine release into the airways.
- Immunology and Microbiology
Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response.
In PLoS Pathogens on 1 February 2014 by Ruckwardt, T. J., Malloy, A. M., et al.
PubMed
CD103+ and CD11b+ populations of CD11c+MHCIIhi murine dendritic cells (DCs) have been shown to carry antigens from the lung through the afferent lymphatics to mediastinal lymph nodes (MLN). We compared the responses of these two DC populations in neonatal and adult mice following intranasal infection with respiratory syncytial virus. The response in neonates was dominated by functionally-limited CD103+ DCs, while CD11b+ DCs were diminished in both number and function compared to adults. Infecting mice at intervals through the first three weeks of life revealed an evolution in DC phenotype and function during early life. Using TCR transgenic T cells with two different specificities to measure the ability of CD103+ DC to induce epitope-specific CD8+ T cell responses, we found that neonatal CD103+ DCs stimulate proliferation in a pattern distinct from adult CD103+ DCs. Blocking CD28-mediated costimulatory signals during adult infection demonstrated that signals from this costimulatory pathway influence the hierarchy of the CD8+ T cell response to RSV, suggesting that limited costimulation provided by neonatal CD103+ DCs is one mechanism whereby neonates generate a distinct CD8+ T cell response from that of adults.
- Immunology and Microbiology
Abrogation of CD40-CD154 signaling impedes the homeostasis of thymic resident regulatory T cells by altering the levels of IL-2, but does not affect regulatory T cell development.
In The Journal of Immunology on 15 August 2012 by Cuss, S. M. & Green, E. A.
PubMed
Identification of costimulatory signals required for murine regulatory T (Treg) cell development relies on measuring the frequency of total thymic Treg cells. However, the thymus contains both resident and newly developed Treg cells; whether such signals target both populations is unknown. In this study, we show that CD40-CD154 blockade specifically targeted thymic resident Treg cells, but not, as was previously believed, newly developed Treg cells. Unlike CD28-CD80/CD86 signals, CD40-CD154 signals were not required for Treg cell precursor development. Instead we demonstrate that homeostatic proliferation of thymic resident Treg cells was dependent on CD40-CD154 signals maintaining IL-2 levels. Furthermore, in newborn mice, where all Treg cells are newly developed, blockade of CD40-CD154 signals had no effect on thymic Treg numbers or their proliferation. Our studies highlight the complexity in the study of thymic Treg cell development due to the heterogeneity of thymic Treg cells.
- Immunology and Microbiology
Structurally identical capsular polysaccharide expressed by intact group B streptococcus versus Streptococcus pneumoniae elicits distinct murine polysaccharide-specific IgG responses in vivo.
In The Journal of Immunology on 1 June 2012 by Arjunaraja, S., Paoletti, L. C., et al.
PubMed
We previously reported distinct differences in the murine in vivo Ig polysaccharide (PS)-specific responses to intact Streptococcus pneumoniae compared with responses to Neisseria meningitidis and that in each case, the bacterial subcapsular domain markedly influences the Ig response to the associated PS. In light of potentially unique contributions of biochemically distinct capsular PS and/or their characteristic attachments to the underlying bacterium, it remains unresolved whether different bacterial subcapsular domains can exert differential effects on PS-specific Ig responses to distinct bacterial pathogens. In this report, we used a mutant strain of group B Streptococcus (Streptococcus agalactiae) type III (GBS-III) that expresses desialylated capsular polysaccharide of GBS-III, biochemically identical to capsular pneumococcal polysaccharide type 14 (PPS14) of Streptococcus pneumoniae (intact inactivated Streptococcus pneumoniae, capsular type 14, Pn14), directly to compare the in vivo PPS14-specific IgG responses to two distinct gram-positive bacteria. Although both GBS-III and Pn14 elicited relatively rapid primary PPS14-specific IgG responses dependent on CD4(+) T cells, B7-dependent costimulation, and CD40-CD40L interactions, only GBS-III induced a highly boosted ICOS-dependent PPS14-specific IgG response after secondary immunization. Of note, priming with Pn14 and boosting with GBS-III, although not isolated PPS14, elicited a similar boosted PPS14-specific IgG response that was dependent on CD4(+) T cells during secondary immunization, indicating that Pn14 primes for memory but, unlike GBS-III, fails to elicit it. The inability of Pn14 to elicit a boosted PPS14-specific IgG response was overcome by coimmunization with unencapsulated GBS-III. Collectively, these data establish that structurally identical capsular PS expressed by two distinct gram-positive extracellular bacteria can indeed elicit distinct PS-specific IgG responses in vivo.