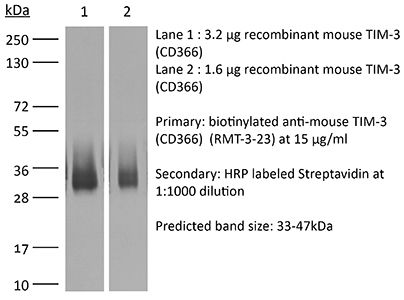
InVivoMAb anti-mouse TIM-3 (CD366)
Product Description
Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse TIM-3 |
| Reported Applications |
in vivo TIM-3 neutralization in vitro TIM-3 blocking Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10949464 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo TIM-3 neutralization
Liu, J. F., et al (2018). "Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer" J Exp Clin Cancer Res 37(1): 44.
PubMed
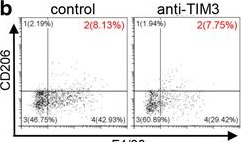
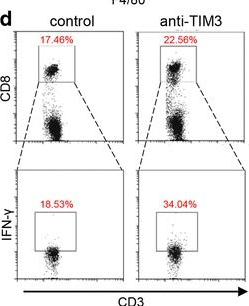
BACKGROUND: T-cell immunoglobulin mucin 3 (TIM3) is a negative immune checkpoint and plays a crucial part in tumor-induced immune suppression. However, the mechanism of TIM3 in regulating immunosuppression in head and neck squamous cell carcinoma (HNSCC) was still not quite clear. METHODS: We carried out the immunohistochemistry staining of HNSCC tissue microarrays. Through quantification of the histoscore, we performed the correlation analysis among the TIM3, Galectin-9, Foxp3, CD68 and CD163. The effects of TIM3 on regulatory T cells (Tregs) and macrophages were detected by utilizing the Tgfbr1/Pten 2cKO HNSCC mouse model. Flow cytometry were used to analysis the percent of Tregs, macrophages and IFN-gamma. RESULTS: We demonstrated the close association among TIM3/Galectin-9 pathway, regulatory T cell marker (Foxp3) and macrophage marker (CD68, CD163) in human HNSCC. In the transgenic HNSCC mouse model, blockade of TIM3 by the anti-TIM3 monoclonal antibody induced a reduction of CD4(+)CD25(+)Foxp3(+) Tregs. Meanwhile, the population of TIM3(+) Tregs was also decreased. However, the population of CD206(+) macrophages was not significantly declined. The increased IFN-gamma production on CD8(+) T cells in anti-TIM3 treatment mice showed that the antitumor immune response was enhanced through suppression of these negative immune factors. CONCLUSIONS: The present study demonstrated that TIM3 was associated with the immunosuppression in HNSCC. And targeting TIM3 can enhance anti-tumor immune response by decreasing Tregs in HNSCC.
in vivo TIM-3 neutralization
Kurtulus, S., et al (2015). "TIGIT predominantly regulates the immune response via regulatory T cells" J Clin Invest. doi : 10.1172/JCI81187.
PubMed
Coinhibitory receptors are critical for the maintenance of immune homeostasis. Upregulation of these receptors on effector T cells terminates T cell responses, while their expression on Tregs promotes their suppressor function. Understanding the function of coinhibitory receptors in effector T cells and Tregs is crucial, as therapies that target coinhibitory receptors are currently at the forefront of treatment strategies for cancer and other chronic diseases. T cell Ig and ITIM domain (TIGIT) is a recently identified coinhibitory receptor that is found on the surface of a variety of lymphoid cells, and its role in immune regulation is just beginning to be elucidated. We examined TIGIT-mediated immune regulation in different murine cancer models and determined that TIGIT marks the most dysfunctional subset of CD8+ T cells in tumor tissue as well as tumor-tissue Tregs with a highly active and suppressive phenotype. We demonstrated that TIGIT signaling in Tregs directs their phenotype and that TIGIT primarily suppresses antitumor immunity via Tregs and not CD8+ T cells. Moreover, TIGIT+ Tregs upregulated expression of the coinhibitory receptor TIM-3 in tumor tissue, and TIM-3 and TIGIT synergized to suppress antitumor immune responses. Our findings provide mechanistic insight into how TIGIT regulates immune responses in chronic disease settings.
in vivo TIM-3 neutralization
Ngiow, S. F., et al (2015). "A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1" Cancer Res 75(18): 3800-3811.
PubMed
Despite successes, thus far, a significant proportion of the patients treated with anti-PD1 antibodies have failed to respond. We use mouse tumor models of anti-PD1 sensitivity and resistance and flow cytometry to assess tumor-infiltrating immune cells immediately after therapy. We demonstrate that the expression levels of T-cell PD1 (PD1(lo)), myeloid, and T-cell PDL1 (PDL1(hi)) in the tumor microenvironment inversely correlate and dictate the efficacy of anti-PD1 mAb and function of intratumor CD8(+) T cells. In sensitive tumors, we reveal a threshold for PD1 downregulation on tumor-infiltrating CD8(+) T cells below which the release of adaptive immune resistance is achieved. In contrast, PD1(hi) T cells in resistant tumors fail to be rescued by anti-PD1 therapy and remain dysfunctional unless intratumor PDL1(lo) immune cells are targeted. Intratumor Tregs are partly responsible for the development of anti-PD1-resistant tumors and PD1(hi) CD8(+) T cells. Our analyses provide a framework to interrogate intratumor CD8(+) T-cell PD1 and immune PDL1 levels and response in human cancer. Cancer Res; 75(18); 3800-11. (c)2015 AACR.
in vivo TIM-3 neutralization
Tripathi, S., et al (2015). "Effect of TIM-3 Blockade on the Immunophenotype and Cytokine Profile of Murine Uterine NK Cells" PLoS One 10(4): e0123439.
PubMed
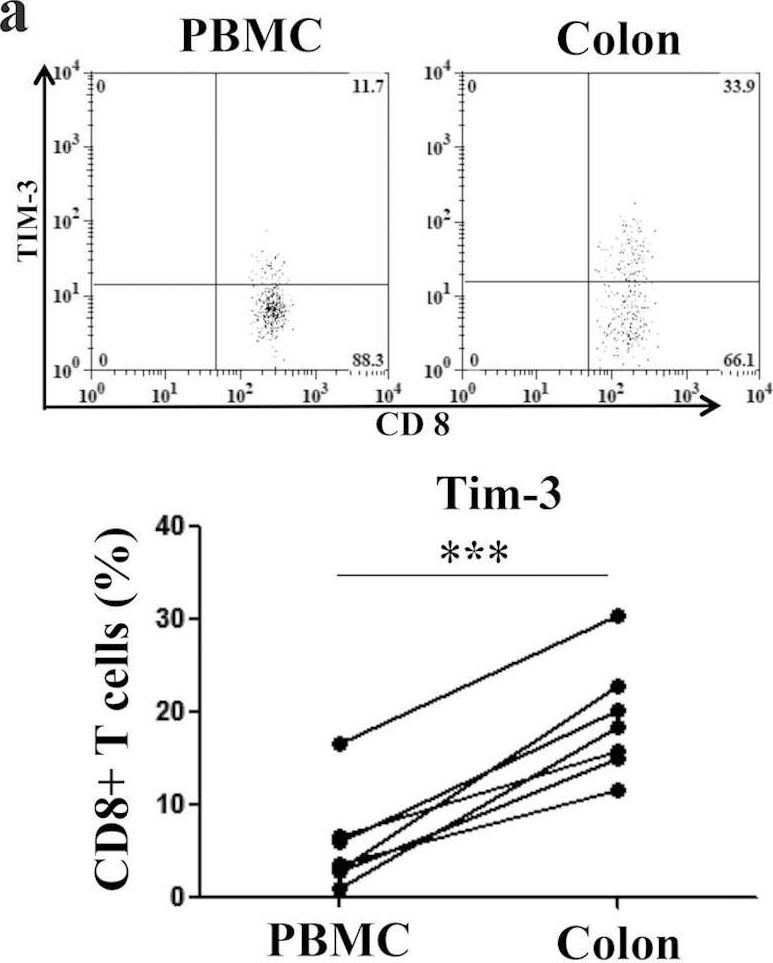
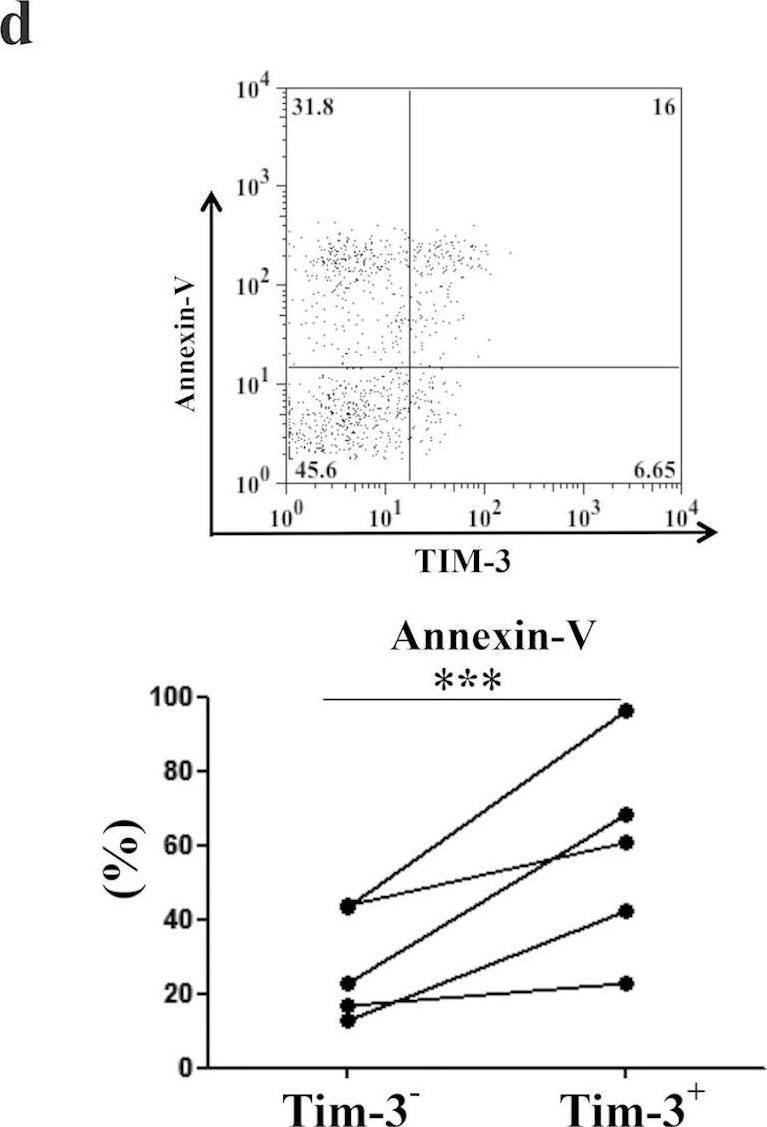
NK cells are the most abundant lymphocyte population in the feto-maternal interface during gestation. The uterine NK cells (uNK) are transient, have a unique immunophenotype and produce a number of cytokines. These cytokines play an important role in establishment and maintenance of vascular remodeling and tolerance associated with successful pregnancy. The uNK cells also express TIM-3 during gestation and blockade of TIM-3 expression results in fetal loss in mice. In this study we determined the effect of TIM-3 blockade on uNK cells. Specifically we observed surface receptor phenotype and cytokine production by uNK cells following TIM-3 blockade. Our results show that TIM-3 plays a role in regulating the uNK cells and contributes to the maintenance of tolerance at the feto-maternal interface.
in vivo TIM-3 neutralization
Mittal, D., et al (2014). "Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor" Cancer Res 74(14): 3652-3658.
PubMed
Adenosine targeting is an attractive new approach to cancer treatment, but no clinical study has yet examined adenosine inhibition in oncology despite the safe clinical profile of adenosine A2A receptor inhibitors (A2ARi) in Parkinson disease. Metastasis is the main cause of cancer-related deaths worldwide, and therefore we have studied experimental and spontaneous mouse models of melanoma and breast cancer metastasis to demonstrate the efficacy and mechanism of a combination of A2ARi in combination with anti-PD-1 monoclonal antibody (mAb). This combination significantly reduces metastatic burden and prolongs the life of mice compared with either monotherapy alone. Importantly, the combination was only effective when the tumor expressed high levels of CD73, suggesting a tumor biomarker that at a minimum could be used to stratify patients that might receive this combination. The mechanism of the combination therapy was critically dependent on NK cells and IFNgamma, and to a lesser extent, CD8(+) T cells and the effector molecule, perforin. Overall, these results provide a strong rationale to use A2ARi with anti-PD-1 mAb for the treatment of minimal residual and metastatic disease.
in vivo TIM-3 neutralization
Dolina, J. S., et al (2014). "Liver-primed CD8+ T cells suppress antiviral adaptive immunity through galectin-9-independent T-cell immunoglobulin and mucin 3 engagement of high-mobility group box 1 in mice" Hepatology 59(4): 1351-1365.
PubMed
The liver is a tolerogenic environment exploited by persistent infections, such as hepatitis B (HBV) and C (HCV) viruses. In a murine model of intravenous hepatotropic adenovirus infection, liver-primed antiviral CD8(+) T cells fail to produce proinflammatory cytokines and do not display cytolytic activity characteristic of effector CD8(+) T cells generated by infection at an extrahepatic, that is, subcutaneous, site. Importantly, liver-generated CD8(+) T cells also appear to have a T-regulatory (Treg) cell function exemplified by their ability to limit proliferation of antigen-specific T-effector (Teff ) cells in vitro and in vivo via T-cell immunoglobulin and mucin 3 (Tim-3) expressed by the CD8(+) Treg cells. Regulatory activity did not require recognition of the canonical Tim-3 ligand, galectin-9, but was dependent on CD8(+) Treg cell-surface Tim-3 binding to the alarmin, high-mobility group box 1 (HMGB-1). CONCLUSION: Virus-specific Tim-3(+) CD8(+) T cells operating through HMGB-1 recognition in the setting of acute and chronic viral infections of the liver may act to dampen hepatic T-cell responses in the liver microenvironment and, as a consequence, limit immune-mediated tissue injury or promote the establishment of persistent infections.
in vitro TIM-3 blocking
Flow Cytometry
Erickson, J. J., et al (2014). "Programmed death-1 impairs secondary effector lung CD8(+) T cells during respiratory virus reinfection" J Immunol 193(10): 5108-5117.
PubMed
Reinfections with respiratory viruses are common and cause significant clinical illness, yet precise mechanisms governing this susceptibility are ill defined. Lung Ag-specific CD8(+) T cells (T(CD8)) are impaired during acute viral lower respiratory infection by the inhibitory receptor programmed death-1 (PD-1). To determine whether PD-1 contributes to recurrent infection, we first established a model of reinfection by challenging B cell-deficient mice with human metapneumovirus (HMPV) several weeks after primary infection, and found that HMPV replicated to high titers in the lungs. A robust secondary effector lung TCD8 response was generated during reinfection, but these cells were more impaired and more highly expressed the inhibitory receptors PD-1, LAG-3, and 2B4 than primary T(CD8). In vitro blockade demonstrated that PD-1 was the dominant inhibitory receptor early after reinfection. In vivo therapeutic PD-1 blockade during HMPV reinfection restored lung T(CD8) effector functions (i.e., degranulation and cytokine production) and enhanced viral clearance. PD-1 also limited the protective efficacy of HMPV epitope-specific peptide vaccination and impaired lung T(CD8) during heterotypic influenza virus challenge infection. Our results indicate that PD-1 signaling may contribute to respiratory virus reinfection and evasion of vaccine-elicited immune responses. These results have important implications for the design of effective vaccines against respiratory viruses.
in vivo TIM-3 neutralization
McGray, A. J., et al (2014). "Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor" Mol Ther 22(1): 206-218.
PubMed
Despite clear evidence of immunogenicity, cancer vaccines only provide a modest clinical benefit. To evaluate the mechanisms that limit tumor regression following vaccination, we have investigated the weak efficacy of a highly immunogenic experimental vaccine using a murine melanoma model. We discovered that the tumor adapts rapidly to the immune attack instigated by tumor-specific CD8+ T cells in the first few days following vaccination, resulting in the upregulation of a complex set of biological networks, including multiple immunosuppressive processes. This rapid adaptation acts to prevent sustained local immune attack, despite continued infiltration by increasing numbers of tumor-specific T cells. Combining vaccination with adoptive transfer of tumor-specific T cells produced complete regression of the treated tumors but did not prevent the adaptive immunosuppression. In fact, the adaptive immunosuppressive pathways were more highly induced in regressing tumors, commensurate with the enhanced level of immune attack. Examination of tumor infiltrating T-cell functionality revealed that the adaptive immunosuppression leads to a progressive loss in T-cell function, even in tumors that are regressing. These novel observations that T cells produced by therapeutic intervention can instigate a rapid adaptive immunosuppressive response within the tumor have important implications for clinical implementation of immunotherapies.
in vivo TIM-3 neutralization
Dietze, K. K., et al (2013). "Combining regulatory T cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8+ T cells and efficiently reduces chronic retroviral loads" PLoS Pathog 9(12): e1003798.
PubMed
Chronic infections with human viruses, such as HIV and HCV, or mouse viruses, such as LCMV or Friend Virus (FV), result in functional exhaustion of CD8(+) T cells. Two main mechanisms have been described that mediate this exhaustion: expression of inhibitory receptors on CD8(+) T cells and expansion of regulatory T cells (Tregs) that suppress CD8(+) T cell activity. Several studies show that blockage of one of these pathways results in reactivation of CD8(+) T cells and partial reduction in chronic viral loads. Using blocking antibodies against PD-1 ligand and Tim-3 and transgenic mice in which Tregs can be selectively ablated, we compared these two treatment strategies and combined them for the first time in a model of chronic retrovirus infection. Blocking inhibitory receptors was more efficient than transient depletion of Tregs in reactivating exhausted CD8(+) T cells and reducing viral set points. However, a combination therapy was superior to any single treatment and further augmented CD8(+) T cell responses and resulted in a sustained reduction in chronic viral loads. These results demonstrate that Tregs and inhibitory receptors are non-overlapping factors in the maintenance of chronic viral infections and that immunotherapies targeting both pathways may be a promising strategy to treat chronic infectious diseases.
in vivo TIM-3 neutralization
Zelinskyy, G., et al (2011). "Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute friend retrovirus infection but are highly cytotoxic and control virus replication" J Immunol 187(7): 3730-3737.
PubMed
It was recently reported that inhibitory molecules such as programmed death-1 (PD-1) were upregulated on CD8(+) T cells during acute Friend retrovirus infection and that the cells were prematurely exhausted and dysfunctional in vitro. The current study confirms that most activated CD8(+) T cells upregulated expression of PD-1 during acute infection and revealed a dichotomy of function between PD-1(hi) and PD-1(lo) subsets. More PD-1(lo) cells produced antiviral cytokines such as IFN-gamma and TNF-alpha, whereas more PD-1(hi) cells displayed characteristics of cytotoxic effectors such as production of granzymes and surface expression of CD107a. Importantly, CD8(+) T cells mediated rapid in vivo cytotoxicity and were critical for control of acute Friend virus replication. Thus, direct ex vivo analyses and in vivo experiments revealed high CD8(+) T cell functionality and indicate that PD-1 expression during acute infection is not a marker of T cell exhaustion.
Product Citations
-
-
Cancer Research
-
Immunology and Microbiology
Identification of anti-TIM-3 based checkpoint inhibitor combinations with activity in immunotherapy refractory melanoma models.
In J Immunother Cancer on 18 August 2025 by Phadke, M. S., Li, J., et al.
PubMed
A significant percentage of melanomas are refractory to immune checkpoint inhibitor (ICI) monotherapies and combinations. As there are currently no effective second-line therapies available for ICI-resistant patients, we sought to identify novel checkpoint inhibitor combinations for future clinical evaluation.
-
-
-
Cancer Research
Inhibitors of oncogenic Kras specifically prime CTLA4 blockade to transcriptionally reprogram Tregs and overcome resistance to suppress pancreas cancer
In bioRxiv on 4 March 2025 by Mahadevan, K. K., Maldonado, A. S., et al.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Dichotomous outcomes of TNFR1 and TNFR2 signaling in NK cell-mediated immune responses during inflammation.
In Nat Commun on 14 November 2024 by McCulloch, T., Rossi, G. R., et al.
PubMed
Natural killer (NK) cell function is regulated by a balance of activating and inhibitory signals. Tumor necrosis factor (TNF) is an inflammatory cytokine ubiquitous across homeostasis and disease, yet its role in regulation of NK cells remains unclear. Here, we find upregulation of the immune checkpoint protein, T cell immunoglobulin and mucin domain 3 (Tim3), is a biomarker of TNF signaling in NK cells during Salmonella Typhimurium infection. In mice with conditional deficiency of either TNF receptor 1 (TNFR1) or TNF receptor 2 (TNFR2) in NK cells, we find TNFR1 limits bacterial clearance whereas TNFR2 promotes it. Mechanistically, via single cell RNA sequencing we find that both TNFR1 and TNFR2 induce the upregulation of Tim3, while TNFR1 accelerates NK cell death but TNFR2 promotes NK cell accumulation and effector function. Our study thus highlights the complex interplay of TNF-based regulation of NK cells by the two TNF receptors during inflammation.
-
-
-
Cancer Research
-
Immunology and Microbiology
Spatial Context of Immune Checkpoints as Predictors of Overall Survival in Patients with Resectable Colorectal Cancer Independent of Standard Tumor-Node-Metastasis Stages.
In Cancer Res Commun on 1 November 2024 by Kong, H., Yang, Q., et al.
PubMed
The identification of specific spatial patterns of immune checkpoint expression that correlate with overall survival in patients with colon cancer suggests a potential prognostic tool for risk stratification and treatment selection. These findings pave the way for the development of novel therapeutic strategies to enhance antitumor immune responses.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Soluble Tim-3 serves as a tumor prognostic marker and therapeutic target for CD8+ T cell exhaustion and anti-PD-1 resistance.
In Cell Rep Med on 20 August 2024 by Chen, C., Zhao, F., et al.
PubMed
Resistance to PD-1 blockade in onco-immunotherapy greatly limits its clinical application. T cell immunoglobulin and mucin domain containing-3 (Tim-3), a promising immune checkpoint target, is cleaved by ADAM10/17 to produce its soluble form (sTim-3) in humans, potentially becoming involved in anti-PD-1 resistance. Herein, serum sTim-3 upregulation was observed in non-small cell lung cancer (NSCLC) and various digestive tumors. Notably, serum sTim-3 is further upregulated in non-responding patients undergoing anti-PD-1 therapy for NSCLC and anti-PD-1-resistant cholangiocarcinoma patients. Furthermore, sTim-3 overexpression facilitates tumor progression and confers anti-PD-1 resistance in multiple tumor mouse models. Mechanistically, sTim-3 induces terminal T cell exhaustion and attenuates CD8+ T cell response to PD-1 blockade through carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1). Moreover, the ADAM10 inhibitor GI254023X, which blocks sTim-3 production, reduces tumor progression in Tim-3 humanized mice and reverses anti-PD-1 resistance in human tumor-infiltrating lymphocytes (TILs). Overall, human sTim-3 holds great predictive and therapeutic potential in onco-immunotherapy.
-
-
-
Mus musculus (Mouse)
-
Epithelium/imcDC2 axis facilitates the resistance of neoadjuvant anti-PD-1 in human NSCLC.
In J Immunother Cancer on 12 August 2024 by Chen, Y., Shao, Z., et al.
PubMed
Therapeutic resistance is a main obstacle to achieve long-term benefits from immune checkpoint inhibitors. The underlying mechanism of neoadjuvant anti-PD-1 resistance remains unclear.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Identification and Validation of a Necroptosis-Related Prognostic Model in Tumor Recurrence and Tumor Immune Microenvironment in Breast Cancer Management.
In J Inflamm Res on 31 July 2024 by Wang, X., Chen, Z., et al.
PubMed
Breast cancer is the leading cause of cancer-related death in women. Necroptosis, a form of programmed necrotic cell death, occurs in many solid tumors, including breast cancer, and influences anti-tumor immunity. The role of necroptosis in managing breast cancer recurrence remains unclear.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
TIM-3 blockade enhances ex vivo stimulated allogeneic NK cell therapy for relapsed murine neuroblastoma after hematopoietic cell transplant
In bioRxiv on 12 July 2024 by Quamine, A., Mohrdieck, N. R., et al.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Combining toll-like receptor agonists with immune checkpoint blockade affects antitumor vaccine efficacy.
In J Immunother Cancer on 3 May 2024 by Jeon, D., Hill, E., et al.
PubMed
T cell checkpoint receptors are expressed when T cells are activated, and modulation of the expression or signaling of these receptors can alter the function of T cells and their antitumor efficacy. We previously found that T cells activated with cognate antigen had increases in the expression of PD-1, and this was attenuated in the presence of multiple toll-like receptor (TLR) agonists, notably TLR3 plus TLR9. In the current report, we sought to investigate whether combining TLR agonists with immune checkpoint blockade can further augment vaccine-mediated T cell antitumor immunity in murine tumor models.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Neuroscience
-
Cancer Research
TIM-3 blockade in diffuse intrinsic pontine glioma models promotes tumor regression and antitumor immune memory.
In Cancer Cell on 13 November 2023 by Ausejo-Mauleon, I., Labiano, S., et al.
PubMed
Diffuse intrinsic pontine glioma (DIPG) is an aggressive brain stem tumor and the leading cause of pediatric cancer-related death. To date, these tumors remain incurable, underscoring the need for efficacious therapies. In this study, we demonstrate that the immune checkpoint TIM-3 (HAVCR2) is highly expressed in both tumor cells and microenvironmental cells, mainly microglia and macrophages, in DIPG. We show that inhibition of TIM-3 in syngeneic models of DIPG prolongs survival and produces long-term survivors free of disease that harbor immune memory. This antitumor effect is driven by the direct effect of TIM-3 inhibition in tumor cells, the coordinated action of several immune cell populations, and the secretion of chemokines/cytokines that create a proinflammatory tumor microenvironment favoring a potent antitumor immune response. This work uncovers TIM-3 as a bona fide target in DIPG and supports its clinical translation.
-
-
-
Cancer Research
-
Immunology and Microbiology
Interactions of Indoleamine 2,3-dioxygenase-expressing LAMP3+ dendritic cells with CD4+ regulatory T cells and CD8+ exhausted T cells: synergistically remodeling of the immunosuppressive microenvironment in cervical cancer and therapeutic implication
In Cancer Commun (Lond) on 1 November 2023 by Qu, X., Wang, Y., et al.
PubMed
Cervical cancer (CC) is the fourth most common cancer in women worldwide. Although immunotherapy has been applied in clinical practice, its therapeutic efficacy remains far from satisfactory, necessitating further investigation of the mechanism of CC immune remodeling and exploration of novel treatment targets. This study aimed to investigate the mechanism of CC immune remodeling and explore potential therapeutic targets.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
T Cell CEACAM1-TIM-3 Crosstalk Alleviates Liver Transplant Injury in Mice and Humans.
In Gastroenterology on 1 November 2023 by Kojima, H., Kadono, K., et al.
PubMed
Carcinoembryonic antigen-related cell adhesion molecule 1 (CC1) acts through homophilic and heterophilic interactions with T cell immunoglobulin domain and mucin domain-containing protein 3 (TIM-3), which regulates innate immune activation in orthotopic liver transplantation (OLT). We investigated whether cluster of differentiation (CD) 4+ T cell-dependent CC1-TIM-3 crosstalk may affect OLT outcomes in mice and humans.
-
-
-
Cancer Research
-
Immunology and Microbiology
Tumor immunogenicity dictates reliance on TCF1 in CD8+ T cells for response to immunotherapy.
In Cancer Cell on 11 September 2023 by Escobar, G., Tooley, K., et al.
PubMed
Stem-like CD8+ T cells are regulated by T cell factor 1 (TCF1) and are considered requisite for immune checkpoint blockade (ICB) response. However, recent findings indicate that reliance on TCF1+CD8+ T cells for ICB efficacy may differ across tumor contexts. We find that TCF1 is essential for optimal priming of tumor antigen-specific CD8+ T cells and ICB response in poorly immunogenic tumors that accumulate TOX+ dysfunctional T cells, but is dispensable for T cell priming and therapy response in highly immunogenic tumors that efficiently expand transitory effectors. Importantly, improving T cell priming by vaccination or by enhancing antigen presentation on tumors rescues the defective responses of TCF1-deficient CD8+ T cells upon ICB in poorly immunogenic tumors. Our study highlights TCF1's role during the early stages of anti-tumor CD8+ T cell responses with important implications for guiding optimal therapeutic interventions in cancers with low TCF1+CD8+ T cells and low-neo-antigen expression.
-
-
-
Cell Biology
-
Immunology and Microbiology
-
Mus musculus (Mouse)
TIM-3 increases the abundance of type-2 dendritic cells during Leishmania donovani infection by enhancing IL-10 production via STAT3.
In Cell Death Dis on 18 May 2023 by Mishra, M., Yadav, M., et al.
PubMed
The outcome of the disease visceral leishmaniasis (VL), caused by Leishmania donovani (LD), largely relies on the relative dominance of host-protective type-1 T helper (Th1) cell response versus disease-promoting type-2 T helper (Th2) cell response. The Th1 and Th2 responses, in turn, are believed to be elicited by type-1 conventional dendritic cells (cDC1) and type-2 conventional DCs (cDC2), respectively. However, it is still unknown which DC subtype (cDC1 or cDC2) predominates during chronic LD infection and the molecular mechanism governing such occurrence. Here we report that in chronically infected mice, the splenic cDC1-cDC2 balance shifted toward the cDC2 subtype and that the receptor T cell immunoglobulin and mucin protein-3 (TIM-3) expressed by DCs played a key role in mediating this effect. Transfer of TIM-3-silenced DCs in fact prevented the predominance of the cDC2 subtype in mice with chronic LD infection. We also found that LD actually upregulated TIM-3 expression on DCs by triggering a TIM-3-mediated signaling pathway STAT3 (signal transducer and activator of transcription 3)→interleukin (IL)-10→c-Src→transcription factors Ets1, Ets2, USF1, and USF2. Notably, TIM-3 promoted STAT3 activation via a non-receptor tyrosine kinase Btk. Adoptive transfer experiments further demonstrated a critical role for STAT3-driven TIM-3 upregulation on DCs in increasing cDC2 abundance in chronically infected mice, which ultimately aided disease pathogenesis by augmenting Th2 responses. These findings document a new immunoregulatory mechanism contributing to disease pathology during LD infection and define TIM-3 as a key mediator of this process.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Orthogonal cytokine engineering enables novel synthetic effector states escaping canonical exhaustion in tumor-rejecting CD8+ T cells.
In Nat Immunol on 1 May 2023 by Corria-Osorio, J., Carmona, S. J., et al.
PubMed
To date, no immunotherapy approaches have managed to fully overcome T-cell exhaustion, which remains a mandatory fate for chronically activated effector cells and a major therapeutic challenge. Understanding how to reprogram CD8+ tumor-infiltrating lymphocytes away from exhausted effector states remains an elusive goal. Our work provides evidence that orthogonal gene engineering of T cells to secrete an interleukin (IL)-2 variant binding the IL-2Rβγ receptor and the alarmin IL-33 reprogrammed adoptively transferred T cells to acquire a novel, synthetic effector state, which deviated from canonical exhaustion and displayed superior effector functions. These cells successfully overcame homeostatic barriers in the host and led-in the absence of lymphodepletion or exogenous cytokine support-to high levels of engraftment and tumor regression. Our work unlocks a new opportunity of rationally engineering synthetic CD8+ T-cell states endowed with the ability to avoid exhaustion and control advanced solid tumors.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Multiagent Intratumoral Immunotherapy Can Be Effective in A20 Lymphoma Clearance and Generation of Systemic T Cell Immunity.
In Cancers (Basel) on 24 March 2023 by Gilman, K. E., Matiatos, A. P., et al.
PubMed
The use of immunotherapies has shown promise against selective human cancers. Identifying novel combinations of innate and adaptive immune cell-activating agents that can work synergistically to suppress tumor growth and provide additional protection against resistance or recurrence is critical. The A20 murine lymphoma model was used to evaluate the effect of various combination immunotherapies administered intratumorally. We show that single-modality treatment with Poly(I:C) or GM-CSF-secreting allogeneic cells only modestly controls tumor growth, whereas when given together there is an improved benefit, with 50% of animals clearing tumors and surviving long-term. Neither heat nor irradiation of GM-CSF-secreting cells enhanced the response over use of live cells. The use of a TIM-3 inhibitory antibody and an OX40 agonist in combination with Poly(I:C) allowed for improved tumor control, with 90% of animals clearing tumors with or without a combination of GM-CSF-secreting cells. Across all treatment groups, mice rejecting their primary A20 tumors were immune to subsequent challenge with A20, and this longstanding immunity was T-cell dependent. The results herein support the use of combinations of innate and adaptive immune activating agents for immunotherapy against lymphoma and should be investigated in other cancer types.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Targeting T cell checkpoints 41BB and LAG3 and myeloid cell CXCR1/CXCR2 results in antitumor immunity and durable response in pancreatic cancer.
In Nat Cancer on 1 January 2023 by Gulhati, P., Schalck, A., et al.
PubMed
Pancreatic ductal adenocarcinoma (PDAC) is considered non-immunogenic, with trials showing its recalcitrance to PD1 and CTLA4 immune checkpoint therapies (ICTs). Here, we sought to systematically characterize the mechanisms underlying de novo ICT resistance and to identify effective therapeutic options for PDAC. We report that agonist 41BB and antagonist LAG3 ICT alone and in combination, increased survival and antitumor immunity, characterized by modulating T cell subsets with antitumor activity, increased T cell clonality and diversification, decreased immunosuppressive myeloid cells and increased antigen presentation/decreased immunosuppressive capability of myeloid cells. Translational analyses confirmed the expression of 41BB and LAG3 in human PDAC. Since single and dual ICTs were not curative, T cell-activating ICTs were combined with a CXCR1/2 inhibitor targeting immunosuppressive myeloid cells. Triple therapy resulted in durable complete responses. Given similar profiles in human PDAC and the availability of these agents for clinical testing, our findings provide a testable hypothesis for this lethal disease.
-
-
-
Mass Spectrometry
-
Cancer Research
-
Immunology and Microbiology
-
Mass Spectrometry
-
Mus musculus (Mouse)
Stromal Reprogramming by FAK Inhibition Overcomes Radiation Resistance to Allow for Immune Priming and Response to Checkpoint Blockade.
In Cancer Discov on 2 December 2022 by Krisnawan, V. E., Belle, J. I., et al.
PubMed
The effects of radiotherapy (RT) on tumor immunity in pancreatic ductal adenocarcinoma (PDAC) are not well understood. To better understand if RT can prime antigen-specific T-cell responses, we analyzed human PDAC tissues and mouse models. In both settings, there was little evidence of RT-induced T-cell priming. Using in vitro systems, we found that tumor-stromal components, including fibroblasts and collagen, cooperate to blunt RT efficacy and impair RT-induced interferon signaling. Focal adhesion kinase (FAK) inhibition rescued RT efficacy in vitro and in vivo, leading to tumor regression, T-cell priming, and enhanced long-term survival in PDAC mouse models. Based on these data, we initiated a clinical trial of defactinib in combination with stereotactic body RT in patients with PDAC (NCT04331041). Analysis of PDAC tissues from these patients showed stromal reprogramming mirroring our findings in genetically engineered mouse models. Finally, the addition of checkpoint immunotherapy to RT and FAK inhibition in animal models led to complete tumor regression and long-term survival.
-
-
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer.
In Sci Immunol on 9 September 2022 by Mirji, G., Worth, A., et al.
PubMed
The composition of the gut microbiome can control innate and adaptive immunity and has emerged as a key regulator of tumor growth, especially in the context of immune checkpoint blockade (ICB) therapy. However, the underlying mechanisms for how the microbiome affects tumor growth remain unclear. Pancreatic ductal adenocarcinoma (PDAC) tends to be refractory to therapy, including ICB. Using a nontargeted, liquid chromatography-tandem mass spectrometry-based metabolomic screen, we identified the gut microbe-derived metabolite trimethylamine N-oxide (TMAO), which enhanced antitumor immunity to PDAC. Delivery of TMAO intraperitoneally or via a dietary choline supplement to orthotopic PDAC-bearing mice reduced tumor growth, associated with an immunostimulatory tumor-associated macrophage (TAM) phenotype, and activated effector T cell response in the tumor microenvironment. Mechanistically, TMAO potentiated the type I interferon (IFN) pathway and conferred antitumor effects in a type I IFN-dependent manner. Delivering TMAO-primed macrophages intravenously produced similar antitumor effects. Combining TMAO with ICB (anti-PD1 and/or anti-Tim3) in a mouse model of PDAC significantly reduced tumor burden and improved survival beyond TMAO or ICB alone. Last, the levels of bacteria containing CutC (an enzyme that generates trimethylamine, the TMAO precursor) correlated with long-term survival in patients with PDAC and improved response to anti-PD1 in patients with melanoma. Together, our study identifies the gut microbial metabolite TMAO as a driver of antitumor immunity and lays the groundwork for potential therapeutic strategies targeting TMAO.
-
-
-
Immunology and Microbiology
-
Mus musculus (Mouse)
Leishmania donovani Impedes Antileishmanial Immunity by Suppressing Dendritic Cells via the TIM-3 Receptor.
In MBio on 30 August 2022 by Akhtar, M. N., Kumar, S., et al.
PubMed
An immunological hallmark of visceral leishmaniasis (VL), caused by Leishmania donovani, is profound immunosuppression. However, the molecular basis for this immune dysfunction has remained ill defined. Since dendritic cells (DCs) normally initiate antileishmanial immune responses, we investigated whether DCs are dysregulated during L. donovani infection and assessed its role in immunosuppression. Accordingly, we determined the regulatory effect of L. donovani on DCs. Notably, it is still unclear whether L. donovani activates or suppresses DCs. In addition, the molecular mechanism and the relevant receptor (or receptors) mediating the immunoregulatory effect of L. donovani on DCs are largely undefined. Here, we report that L. donovani inhibited DC activation/maturation by transmitting inhibitory signals through the T cell immunoglobulin and mucin protein-3 (TIM-3) receptor and thereby suppressed antileishmanial immune responses. L. donovani in fact triggered TIM-3 phosphorylation in DCs, which in turn recruited and activated a nonreceptor tyrosine kinase, Btk. Btk then inhibited DC activation/maturation by suppressing the NF-κB pathway in an interleukin-10 (IL-10)-dependent manner. Treatment with TIM-3-specific blocking antibody or suppressed expression of TIM-3 or downstream effector Btk made DCs resistant to the inhibitory effects of L. donovani. Adoptive transfer experiments further demonstrated that TIM-3-mediated L. donovani-induced inhibition of DCs plays a crucial role in the suppression of the antileishmanial immune response in vivo. These findings identify TIM-3 as a new regulator of the antileishmanial immune response and demonstrate a unique mechanism for host immunosuppression associated with L. donovani infection. IMPORTANCE Visceral leishmaniasis (VL), a poverty-related disease caused by Leishmania donovani, is ranked by the World Health Organization as the second largest killer parasitic disease in the world. The protective immune response against VL is primarily regulated by dendritic cells (DCs), which upon activation/maturation initiate an antileishmanial immune response. However, it remains obscure whether L. donovani promotes or inhibits DC activation. In addition, the receptor through which L. donovani exerts immunoregulatory effect on DCs is ill defined. Here, we for the first time report that L. donovani inhibits DC activation and maturation via the T cell immunoglobulin and mucin protein-3 (TIM-3) receptor and thereby attenuates the capacity of DCs to trigger antileishmanial immune responses in vivo. In fact, we demonstrate here that suppression of TIM-3 expression in DCs augments antileishmanial immunity. Our study uncovers a unique mechanism by which L. donovani subverts host immune responses and suggests TIM-3 as a potential new target for immunotherapy against VL.
-