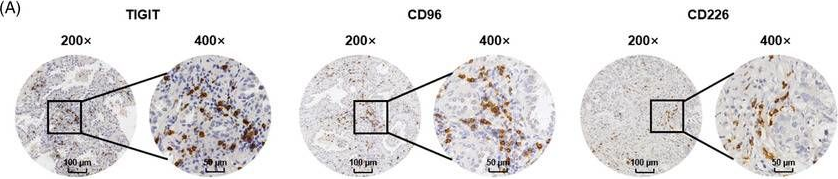
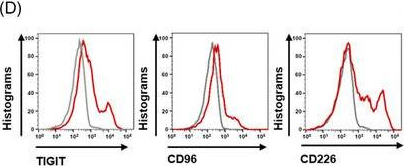
InVivoMAb anti-mouse TIGIT
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse TIGIT |
| Reported Applications |
in vivo TIGIT stimulation Flow cytometry |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_2687797 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo TIGIT stimulation
Schorer, M., et al (2020). "TIGIT limits immune pathology during viral infections" Nat Commun 11(1): 1288.
PubMed
Co-inhibitory pathways have a fundamental function in regulating T cell responses and control the balance between promoting efficient effector functions and restricting immune pathology. The TIGIT pathway has been implicated in promoting T cell dysfunction in chronic viral infection. Importantly, TIGIT signaling is functionally linked to IL-10 expression, which has an effect on both virus control and maintenance of tissue homeostasis. However, whether TIGIT has a function in viral persistence or limiting tissue pathology is unclear. Here we report that TIGIT modulation effectively alters the phenotype and cytokine profile of T cells during influenza and chronic LCMV infection, but does not affect virus control in vivo. Instead, TIGIT has an important effect in limiting immune pathology in peripheral organs by inducing IL-10. Our data therefore identify a function of TIGIT in limiting immune pathology that is independent of viral clearance.
in vivo TIGIT stimulation
Dixon, K. O., et al (2018). "Functional Anti-TIGIT Antibodies Regulate Development of Autoimmunity and Antitumor Immunity" J Immunol 200(8): 3000-3007.
PubMed
Coinhibitory receptors, such as CTLA-4 and PD-1, play a critical role in maintaining immune homeostasis by dampening T cell responses. Recently, they have gained attention as therapeutic targets in chronic disease settings where their dysregulated expression contributes to suppressed immune responses. The novel coinhibitory receptor TIGIT (T cell Ig and ITIM domain) has been shown to play an important role in modulating immune responses in the context of autoimmunity and cancer. However, the molecular mechanisms by which TIGIT modulates immune responses are still insufficiently understood. We have generated a panel of monoclonal anti-mouse TIGIT Abs that show functional properties in mice in vivo and can serve as important tools to study the underlying mechanisms of TIGIT function. We have identified agonistic as well as blocking anti-TIGIT Ab clones that are capable of modulating T cell responses in vivo. Administration of either agonist or blocking anti-TIGIT Abs modulated autoimmune disease severity whereas administration of blocking anti-TIGIT Abs synergized with anti-PD-1 Abs to affect partial or even complete tumor regression. The Abs presented in this study can thus serve as important tools for detailed analysis of TIGIT function in different disease settings and the knowledge gained will provide valuable insight for the development of novel therapeutic approaches targeting TIGIT.
Flow Cytometry
Burton, B. R., et al (2014). "Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy" Nat Commun 5: 4741.
PubMed
Antigen-specific immunotherapy combats autoimmunity or allergy by reinstating immunological tolerance to target antigens without compromising immune function. Optimization of dosing strategy is critical for effective modulation of pathogenic CD4(+) T-cell activity. Here we report that dose escalation is imperative for safe, subcutaneous delivery of the high self-antigen doses required for effective tolerance induction and elicits anergic, interleukin (IL)-10-secreting regulatory CD4(+) T cells. Analysis of the CD4(+) T-cell transcriptome, at consecutive stages of escalating dose immunotherapy, reveals progressive suppression of transcripts positively regulating inflammatory effector function and repression of cell cycle pathways. We identify transcription factors, c-Maf and NFIL3, and negative co-stimulatory molecules, LAG-3, TIGIT, PD-1 and TIM-3, which characterize this regulatory CD4(+) T-cell population and whose expression correlates with the immunoregulatory cytokine IL-10. These results provide a rationale for dose escalation in T-cell-directed immunotherapy and reveal novel immunological and transcriptional signatures as surrogate markers of successful immunotherapy.
Flow Cytometry
Chan, C. J., et al (2014). "The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions" Nat Immunol 15(5): 431-438.
PubMed
CD96, CD226 (DNAM-1) and TIGIT belong to an emerging family of receptors that interact with nectin and nectin-like proteins. CD226 activates natural killer (NK) cell-mediated cytotoxicity, whereas TIGIT reportedly counterbalances CD226. In contrast, the role of CD96, which shares the ligand CD155 with CD226 and TIGIT, has remained unclear. In this study we found that CD96 competed with CD226 for CD155 binding and limited NK cell function by direct inhibition. As a result, Cd96(-/-) mice displayed hyperinflammatory responses to the bacterial product lipopolysaccharide (LPS) and resistance to carcinogenesis and experimental lung metastases. Our data provide the first description, to our knowledge, of the ability of CD96 to negatively control cytokine responses by NK cells. Blocking CD96 may have applications in pathologies in which NK cells are important.
Flow Cytometry
Foks, A. C., et al (2013). "Agonistic anti-TIGIT treatment inhibits T cell responses in LDLr deficient mice without affecting atherosclerotic lesion development" PLoS One 8(12): e83134.
PubMed
OBJECTIVE: Co-stimulatory and co-inhibitory molecules are mainly expressed on T cells and antigen presenting cells and strongly orchestrate adaptive immune responses. Whereas co-stimulatory molecules enhance immune responses, signaling via co-inhibitory molecules dampens the immune system, thereby showing great therapeutic potential to prevent cardiovascular diseases. Signaling via co-inhibitory T cell immunoglobulin and ITIM domain (TIGIT) directly inhibits T cell activation and proliferation, and therefore represents a novel therapeutic candidate to specifically dampen pro-atherogenic T cell reactivity. In the present study, we used an agonistic anti-TIGIT antibody to determine the effect of excessive TIGIT-signaling on atherosclerosis. METHODS AND RESULTS: TIGIT was upregulated on CD4(+) T cells isolated from mice fed a Western-type diet in comparison with mice fed a chow diet. Agonistic anti-TIGIT suppressed T cell activation and proliferation both in vitro and in vivo. However, agonistic anti-TIGIT treatment of LDLr(-/-) mice fed a Western-type diet for 4 or 8 weeks did not affect atherosclerotic lesion development in comparison with PBS and Armenian Hamster IgG treatment. Furthermore, elevated percentages of dendritic cells were observed in the blood and spleen of agonistic anti-TIGIT-treated mice. Additionally, these cells showed an increased activation status but decreased IL-10 production. CONCLUSIONS: Despite the inhibition of splenic T cell responses, agonistic anti-TIGIT treatment does not affect initial atherosclerosis development, possibly due to increased activity of dendritic cells.
Flow Cytometry
Joller, N., et al (2011). "Cutting edge: TIGIT has T cell-intrinsic inhibitory functions" J Immunol 186(3): 1338-1342.
PubMed
Costimulatory molecules regulate the functional outcome of T cell activation, and disturbance of the balance between activating and inhibitory signals results in increased susceptibility to infection or the induction of autoimmunity. Similar to the well-characterized CD28/CTLA-4 costimulatory pathway, a newly emerging pathway consisting of CD226 and T cell Ig and ITIM domain (TIGIT) has been associated with susceptibility to multiple autoimmune diseases. In this study, we examined the role of the putative coinhibitory molecule TIGIT and show that loss of TIGIT in mice results in hyperproliferative T cell responses and increased susceptibility to autoimmunity. TIGIT is thought to indirectly inhibit T cell responses by the induction of tolerogenic dendritic cells. By generating an agonistic anti-TIGIT Ab, we demonstrate that TIGIT can inhibit T cell responses directly independent of APCs. Microarray analysis of T cells stimulated with agonistic anti-TIGIT Ab revealed that TIGIT can act directly on T cells by attenuating TCR-driven activation signals.
Product Citations
-
-
Immunohistochemistry
-
Immunology and Microbiology
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Immunology and Microbiology
-
Flow cytometry/Cell sorting
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Flow cytometry/Cell sorting
-
Immunology and Microbiology
TIGIT blockade improves anti-Mycobacterium tuberculosis immunity.
In PLoS Pathog on 1 June 2025 by Zhou, J., Yang, Q., et al.
PubMed
Despite the therapeutic benefit of immune checkpoint blockade in cancers, there is no consensus on its effect in infectious diseases. Here we investigated whether blocking the immune checkpoint T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains (TIGIT) increases T cell immunity in active Mycobacterium tuberculosis infection. TIGIT expression in both peripheral blood and lung lesions in tuberculosis patients was assessed, and the correlation with clinical features analyzed. The functional status of TIGIT+ and TIGIT-CD8+ T cell subsets in tuberculosis patients was analyzed by flow cytometry and transcriptome analysis. To investigate the regulatory effect of TIGIT, the function of CD8+ T cells in tuberculosis patients and bacterial load in a tuberculosis mouse model were assessed after in vitro and in vivo TIGIT blockade. In active tuberculosis patients, TIGIT expression on CD8+ T cells in the peripheral blood was significantly upregulated and positively correlated with disease severity. TIGIT expression in lung lesions was significantly higher in patients with pulmonary tuberculosis than in patients infected with other pathogens. TIGIT+CD8+ T cells exhibited higher activation and differentiation levels, increased expression levels of cytokines and cytotoxic molecules, and showed gene expression features of natural killer-like cytotoxic effector CD8+ T cells. TIGIT blockade increased the ability of human CD8+ T cells to produce effector molecules and kill intracellular bacteria in vitro. Importantly, blocking TIGIT reduced lung bacterial burden in mice infected with M. tuberculosis. The findings reveal that in active tuberculosis patients, activated CD8+ T cells express TIGIT and blocking TIGIT enhances CD8+ T cell function and promotes clearance of M. tuberculosis. The findings also suggest that TIGIT limits T cell immunity in tuberculosis and implicate TIGIT blockade as a novel strategy for tuberculosis therapy.
-
-
-
Cancer Research
NECTIN4 regulates the cell surface expression of CD155 in non-small cell lung cancer cells and induces tumor resistance to PD-1 inhibitors.
In Cancer Immunol Immunother on 20 May 2025 by Mizusaki, S., Yoneshima, Y., et al.
PubMed
The development of immune checkpoint inhibitors has changed treatment strategies for some patients with non-small cell lung cancer (NSCLC). However, resistance remains a major problem, requiring the elucidation of resistance mechanisms, which might aid the development of novel therapeutic strategies. The upregulation of CD155, a primary ligand of the immune checkpoint receptor TIGIT, has been implicated in a mechanism of resistance to PD-1/PD-L1 inhibitors, and it is therefore important to characterize the mechanisms underlying the regulation of CD155 expression in tumor cells. The aim of this study was to identify a Nectin that might regulate CD155 expression in NSCLC and affect anti-tumor immune activity. In this study, we demonstrated that NECTIN4 regulated the cell surface expression and stabilization of CD155 by interacting and co-localizing with CD155 on the cell surface. In a syngeneic mouse model, NECTIN4-overexpressing cells exhibited increased cell surface CD155 and resistance to anti-PD-1 antibodies. Of note, combination therapy with anti-PD-1 and anti-TIGIT antibodies significantly suppressed tumor growth. These findings provide new insights into the mechanisms of resistance to anti-PD-1 antibodies and suggest that NECTIN4 could serve as a valuable marker in therapeutic strategies targeting TIGIT.
-
-
-
Western Blotting
-
Biochemistry and Molecular biology
-
Cell Biology
-
Genetics
-
Immunology and Microbiology
TIGIT deficiency promotes autoreactive CD4+ T-cell responses through a metabolic‒epigenetic mechanism in autoimmune myositis.
In Nat Commun on 15 May 2025 by Lai, Y., Wang, S., et al.
PubMed
Polymyositis (PM) is a systemic autoimmune disease characterized by muscular inflammatory infiltrates and degeneration. T-cell immunoreceptor with Ig and ITIM domains (TIGIT) contributes to immune tolerance by inhibiting T cell-mediated autoimmunity. Here, we show that a reduced expression of TIGIT in CD4+ T cells from patients with PM promotes these cells' differentiation into Th1 and Th17 cells, which could be rescued by TIGIT overexpression. Knockout of TIGIT enhances muscle inflammation in a mouse model of experimental autoimmune myositis. Mechanistically, we find that TIGIT deficiency enhances CD28-mediated PI3K/AKT/mTOR co-stimulatory pathway, which promotes glucose oxidation, citrate production, and increased cytosolic acetyl-CoA levels, ultimately inducing epigenetic reprogramming via histone acetylation. Importantly, pharmacological inhibition of histone acetylation suppresses the differentiation of Th1 and Th17 cells, alleviating muscle inflammation. Thus, our findings reveal a mechanism by which TIGIT directly affects the differentiation of Th1 and Th17 T cells through metabolic‒epigenetic reprogramming, with important implications for treating systemic autoimmune diseases.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
Cancer cell plasticity defines response to immunotherapy in cutaneous squamous cell carcinoma.
In Nat Commun on 24 June 2024 by Lorenzo-Sanz, L., Lopez-Cerda, M., et al.
PubMed
Immune checkpoint blockade (ICB) approaches have changed the therapeutic landscape for many tumor types. However, half of cutaneous squamous cell carcinoma (cSCC) patients remain unresponsive or develop resistance. Here, we show that, during cSCC progression in male mice, cancer cells acquire epithelial/mesenchymal plasticity and change their immune checkpoint (IC) ligand profile according to their features, dictating the IC pathways involved in immune evasion. Epithelial cancer cells, through the PD-1/PD-L1 pathway, and mesenchymal cancer cells, through the CTLA-4/CD80 and TIGIT/CD155 pathways, differentially block antitumor immune responses and determine the response to ICB therapies. Accordingly, the anti-PD-L1/TIGIT combination is the most effective strategy for blocking the growth of cSCCs that contain both epithelial and mesenchymal cancer cells. The expression of E-cadherin/Vimentin/CD80/CD155 proteins in cSCC, HNSCC and melanoma patient samples predicts response to anti-PD-1/PD-L1 therapy. Collectively, our findings indicate that the selection of ICB therapies should take into account the epithelial/mesenchymal features of cancer cells.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
-
Immunology and Microbiology
The diversity of inhibitory receptor co-expression patterns of exhausted CD8+ T cells in oropharyngeal carcinoma.
In iScience on 17 May 2024 by Rao, Y., Qiu, K., et al.
PubMed
Exhausted CD8+ T cells (Texs) are characterized by the expression of various inhibitory receptors (IRs), whereas the functional attributes of these co-expressed IRs remain limited. Here, we systematically characterized the diversity of IR co-expression patterns in Texs from both human oropharyngeal squamous cell carcinoma (OPSCC) tissues and syngeneic OPSCC model. Nearly 60% of the Texs population co-expressed two or more IRs, and the number of co-expressed IRs was positively associated with superior exhaustion and cytotoxicity phenotypes. In OPSCC patients, programmed cell death-1 (PD-1) blockade significantly enhanced PDCD1-based co-expression with other IR genes, whereas dual blockades of PD-1 and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) significantly upregulated CTLA4-based co-expression with other IR genes. Collectively, our findings demonstrate that highly diverse IR co-expression is a leading feature of Texs and represents their functional states, which might provide essential clues for the rational selection of immune checkpoint inhibitors in treating OPSCC.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Combining toll-like receptor agonists with immune checkpoint blockade affects antitumor vaccine efficacy.
In J Immunother Cancer on 3 May 2024 by Jeon, D., Hill, E., et al.
PubMed
T cell checkpoint receptors are expressed when T cells are activated, and modulation of the expression or signaling of these receptors can alter the function of T cells and their antitumor efficacy. We previously found that T cells activated with cognate antigen had increases in the expression of PD-1, and this was attenuated in the presence of multiple toll-like receptor (TLR) agonists, notably TLR3 plus TLR9. In the current report, we sought to investigate whether combining TLR agonists with immune checkpoint blockade can further augment vaccine-mediated T cell antitumor immunity in murine tumor models.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
HSP47 Destabilizes CD155 Through TRAF2 in Synergistic Anti-TIGIT Treatment of Osteosarcoma
In Research Square on 20 February 2024 by Ye, Z., Mou, H., et al.
-
-
-
Cancer Research
-
Immunology and Microbiology
Targeting TIGIT for cancer immunotherapy: recent advances and future directions.
In Biomark Res on 16 January 2024 by Zhang, P., Liu, X., et al.
PubMed
As a newly identified checkpoint, T cell immunoreceptor with immunoglobulin and tyrosine-based inhibitory motif (ITIM) domain (TIGIT) is highly expressed on CD4+ T cells, CD8+ T cells, natural killer (NK) cells, regulatory T cells (Tregs), and tumor-infiltrating lymphocytes (TILs). TIGIT has been associated with NK cell exhaustion in vivo and in individuals with various cancers. It not only modulates NK cell survival but also mediates T cell exhaustion. As the primary ligand of TIGIT in humans, CD155 may be the main target for immunotherapy due to its interaction with TIGIT. It has been found that the anti-programmed cell death protein 1 (PD-1) treatment response in cancer immunotherapy is correlated with CD155 but not TIGIT. Anti-TIGIT alone and in combination with anti-PD-1 agents have been tested for cancer immunotherapy. Although two clinical studies on advanced lung cancer had positive results, the TIGIT-targeted antibody, tiragolumab, recently failed in two new trials. In this review, we highlight the current developments on TIGIT for cancer immunotherapy and discuss the characteristics and functions of TIGIT.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
-
Cancer Research
-
Flow cytometry/Cell sorting
-
Flow cytometry/Cell sorting
Combining TIGIT blockade with IL-15 stimulation is a promising immunotherapy strategy for lung adenocarcinoma.
In Clin Transl Med on 1 January 2024 by Luo, B., Sun, Y., et al.
PubMed
T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain (TIGIT) is an immune checkpoint molecule that suppresses CD8+ T-cell function in cancer. However, the expression profile and functional significance of TIGIT in the immune microenvironment of lung adenocarcinoma (LUAD) remain elusive. Interleukin (IL)-15 has emerged as a promising candidate for enhancing CD8+ T-cell mediated tumour eradication. Exploring therapeutic strategies that combine IL-15 with TIGIT blockade in LUAD is warranted.
-
-
-
Cancer Research
-
Immunology and Microbiology
Interactions of Indoleamine 2,3-dioxygenase-expressing LAMP3+ dendritic cells with CD4+ regulatory T cells and CD8+ exhausted T cells: synergistically remodeling of the immunosuppressive microenvironment in cervical cancer and therapeutic implication
In Cancer Commun (Lond) on 1 November 2023 by Qu, X., Wang, Y., et al.
PubMed
Cervical cancer (CC) is the fourth most common cancer in women worldwide. Although immunotherapy has been applied in clinical practice, its therapeutic efficacy remains far from satisfactory, necessitating further investigation of the mechanism of CC immune remodeling and exploration of novel treatment targets. This study aimed to investigate the mechanism of CC immune remodeling and explore potential therapeutic targets.
-
-
-
Immunohistochemistry
-
Mus musculus (Mouse)
-
Cancer Research
Peptide-based PET imaging agent of tumor TIGIT expression.
In EJNMMI Res on 2 May 2023 by Weng, D., Guo, R., et al.
PubMed
Accumulating studies have demonstrated that elevated TIGIT expression in tumor microenvironment correlates with better therapeutic response to TIGIT-based immunotherapy in pre-clinical studies. Therefore, a non-invasive method to detect tumor TIGIT expression is crucial to predict the therapeutic effect.
-
-
-
Cancer Research
-
Immunology and Microbiology
-
Mus musculus (Mouse)
T cell-derived interleukin-22 drives the expression of CD155 by cancer cells to suppress NK cell function and promote metastasis.
In Immunity on 10 January 2023 by Briukhovetska, D., Suarez-Gosalvez, J., et al.
PubMed
Although T cells can exert potent anti-tumor immunity, a subset of T helper (Th) cells producing interleukin-22 (IL-22) in breast and lung tumors is linked to dismal patient outcome. Here, we examined the mechanisms whereby these T cells contribute to disease. In murine models of lung and breast cancer, constitutional and T cell-specific deletion of Il22 reduced metastases without affecting primary tumor growth. Deletion of the IL-22 receptor on cancer cells decreases metastasis to a degree similar to that seen in IL-22-deficient mice. IL-22 induced high expression of CD155, which bound to the activating receptor CD226 on NK cells. Excessive activation led to decreased amounts of CD226 and functionally impaired NK cells, which elevated the metastatic burden. IL-22 signaling was also associated with CD155 expression in human datasets and with poor patient outcomes. Taken together, our findings reveal an immunosuppressive circuit activated by T cell-derived IL-22 that promotes lung metastasis.
-