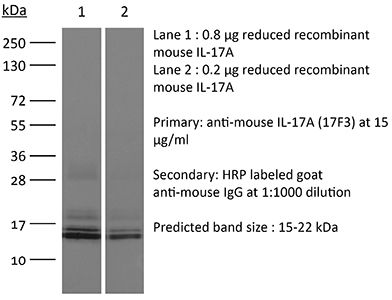
InVivoMAb anti-mouse/rat IL-17A
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Mouse IL-17A cross-linked to OVA |
| Reported Applications |
in vivo IL-17A neutralization ELISA |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
≤1EU/mg (≤0.001EU/μg) Determined by LAL assay |
| Purity |
≥95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_10950102 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
in vivo IL-17A neutralization
Faraco, G., et al (2018). "Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response" Nat Neurosci 21(2): 240-249.
PubMed
A diet rich in salt is linked to an increased risk of cerebrovascular diseases and dementia, but it remains unclear how dietary salt harms the brain. We report that, in mice, excess dietary salt suppresses resting cerebral blood flow and endothelial function, leading to cognitive impairment. The effect depends on expansion of TH17 cells in the small intestine, resulting in a marked increase in plasma interleukin-17 (IL-17). Circulating IL-17, in turn, promotes endothelial dysfunction and cognitive impairment by the Rho kinase-dependent inhibitory phosphorylation of endothelial nitric oxide synthase and reduced nitric oxide production in cerebral endothelial cells. The findings reveal a new gut-brain axis linking dietary habits to cognitive impairment through a gut-initiated adaptive immune response compromising brain function via circulating IL-17. Thus, the TH17 cell-IL-17 pathway is a putative target to counter the deleterious brain effects induced by dietary salt and other diseases associated with TH17 polarization.
in vivo IL-17A neutralization
Xiong, H., et al (2016). "Innate Lymphocyte/Ly6C Monocyte Crosstalk Promotes Klebsiella Pneumoniae Clearance" Cell. doi : 10.1016/j.cell.2016.03.017.
PubMed
Increasing antibiotic resistance among bacterial pathogens has rendered some infections untreatable with available antibiotics. Klebsiella pneumoniae, a bacterial pathogen that has acquired high-level antibiotic resistance, is a common cause of pulmonary infections. Optimal clearance of K. pneumoniae from the host lung requires TNF and IL-17A. Herein, we demonstrate that inflammatory monocytes are rapidly recruited to the lungs of K. pneumoniae-infected mice and produce TNF, which markedly increases the frequency of IL-17-producing innate lymphoid cells. While pulmonary clearance of K. pneumoniae is preserved in neutrophil-depleted mice, monocyte depletion or TNF deficiency impairs IL-17A-dependent resolution of pneumonia. Monocyte-mediated bacterial uptake and killing is enhanced by ILC production of IL-17A, indicating that innate lymphocytes engage in a positive-feedback loop with monocytes that promotes clearance of pneumonia. Innate immune defense against a highly antibiotic-resistant bacterial pathogen depends on crosstalk between inflammatory monocytes and innate lymphocytes that is mediated by TNF and IL-17A.
in vivo IL-17A neutralization
Coffelt, S. B., et al (2015). "IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis" Nature 522(7556): 345-348.
PubMed
Metastatic disease remains the primary cause of death for patients with breast cancer. The different steps of the metastatic cascade rely on reciprocal interactions between cancer cells and their microenvironment. Within this local microenvironment and in distant organs, immune cells and their mediators are known to facilitate metastasis formation. However, the precise contribution of tumour-induced systemic inflammation to metastasis and the mechanisms regulating systemic inflammation are poorly understood. Here we show that tumours maximize their chance of metastasizing by evoking a systemic inflammatory cascade in mouse models of spontaneous breast cancer metastasis. We mechanistically demonstrate that interleukin (IL)-1beta elicits IL-17 expression from gamma delta (gammadelta) T cells, resulting in systemic, granulocyte colony-stimulating factor (G-CSF)-dependent expansion and polarization of neutrophils in mice bearing mammary tumours. Tumour-induced neutrophils acquire the ability to suppress cytotoxic T lymphocytes carrying the CD8 antigen, which limit the establishment of metastases. Neutralization of IL-17 or G-CSF and absence of gammadelta T cells prevents neutrophil accumulation and downregulates the T-cell-suppressive phenotype of neutrophils. Moreover, the absence of gammadelta T cells or neutrophils profoundly reduces pulmonary and lymph node metastases without influencing primary tumour progression. Our data indicate that targeting this novel cancer-cell-initiated domino effect within the immune system–the gammadelta T cell/IL-17/neutrophil axis–represents a new strategy to inhibit metastatic disease.
in vivo IL-17A neutralization
Naik, S., et al (2015). "Commensal-dendritic-cell interaction specifies a unique protective skin immune signature" Nature 520(7545): 104-108.
PubMed
The skin represents the primary interface between the host and the environment. This organ is also home to trillions of microorganisms that play an important role in tissue homeostasis and local immunity. Skin microbial communities are highly diverse and can be remodelled over time or in response to environmental challenges. How, in the context of this complexity, individual commensal microorganisms may differentially modulate skin immunity and the consequences of these responses for tissue physiology remains unclear. Here we show that defined commensals dominantly affect skin immunity and identify the cellular mediators involved in this specification. In particular, colonization with Staphylococcus epidermidis induces IL-17A(+) CD8(+) T cells that home to the epidermis, enhance innate barrier immunity and limit pathogen invasion. Commensal-specific T-cell responses result from the coordinated action of skin-resident dendritic cell subsets and are not associated with inflammation, revealing that tissue-resident cells are poised to sense and respond to alterations in microbial communities. This interaction may represent an evolutionary means by which the skin immune system uses fluctuating commensal signals to calibrate barrier immunity and provide heterologous protection against invasive pathogens. These findings reveal that the skin immune landscape is a highly dynamic environment that can be rapidly and specifically remodelled by encounters with defined commensals, findings that have profound implications for our understanding of tissue-specific immunity and pathologies.
in vivo IL-17A neutralization
Sell, S., et al (2015). "Control of murine cytomegalovirus infection by gammadelta T cells" PLoS Pathog 11(2): e1004481.
PubMed
Infections with cytomegalovirus (CMV) can cause severe disease in immunosuppressed patients and infected newborns. Innate as well as cellular and humoral adaptive immune effector functions contribute to the control of CMV in immunocompetent individuals. None of the innate or adaptive immune functions are essential for virus control, however. Expansion of gammadelta T cells has been observed during human CMV (HCMV) infection in the fetus and in transplant patients with HCMV reactivation but the protective function of gammadelta T cells under these conditions remains unclear. Here we show for murine CMV (MCMV) infections that mice that lack CD8 and CD4 alphabeta-T cells as well as B lymphocytes can control a MCMV infection that is lethal in RAG-1(-/-) mice lacking any T- and B-cells. gammadelta T cells, isolated from infected mice can kill MCMV infected target cells in vitro and, importantly, provide long-term protection in infected RAG-1(-/-) mice after adoptive transfer. gammadelta T cells in MCMV infected hosts undergo a prominent and long-lasting phenotypic change most compatible with the view that the majority of the gammadelta T cell population persists in an effector/memory state even after resolution of the acute phase of the infection. A clonotypically focused Vgamma1 and Vgamma2 repertoire was observed at later stages of the infection in the organs where MCMV persists. These findings add gammadelta T cells as yet another protective component to the anti-CMV immune response. Our data provide clear evidence that gammadelta T cells can provide an effective control mechanism of acute CMV infections, particularly when conventional adaptive immune mechanisms are insufficient or absent, like in transplant patient or in the developing immune system in utero. The findings have implications in the stem cell transplant setting, as antigen recognition by gammadelta T cells is not MHC-restricted and dual reactivity against CMV and tumors has been described.
in vivo IL-17A neutralization
Ermann, J., et al (2014). "Nod/Ripk2 signaling in dendritic cells activates IL-17A-secreting innate lymphoid cells and drives colitis in T-bet-/-.Rag2-/- (TRUC) mice" Proc Natl Acad Sci U S A 111(25): E2559-2566.
PubMed
T-bet(-/-).Rag2(-/-) (TRUC) mice spontaneously develop microbiota-driven, TNF-mediated large bowel inflammation that resembles human ulcerative colitis. We show here that IL-23 and IL-1-dependent secretion of IL-17A by innate lymphoid cells (ILCs; defined as CD45(+)lin(-)Thy1(hi)NKp46(-)) is a second critical pathway in this model. Using an in vitro coculture system of bone marrow-derived dendritic cells (DCs) and freshly isolated FACS-purified ILCs, we demonstrate that IL-23 and IL-1 secreted by DCs in response to microbial stimulation work together to induce IL-17A production by ILCs. TNF is not required for IL-17A secretion by ILCs in vitro but synergizes with IL-17A to induce the expression of neutrophil-attracting chemokines. Upstream, activation of the IL-23/IL-17A axis is regulated by nucleotide-binding oligomerization domain containing (Nod)/receptor-interacting serine-threonine kinase 2 (Ripk2) signals in DCs. Genetic ablation of the Nod/Ripk2 signaling pathway protects TRUC mice from developing colitis without affecting the colitogenicity of the intestinal microbiota. Our data provide insight into the complex network of interactions between IL-17A-secreting ILCs and other components of the innate immune system in the development of colitis.
in vivo IL-17A neutralization
Khmaladze, I., et al (2014). "Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice" Proc Natl Acad Sci U S A 111(35): E3669-3678.
PubMed
Psoriasis (Ps) and psoriasis arthritis (PsA) are poorly understood common diseases, induced by unknown environmental factors, affecting skin and articular joints. A single i.p. exposure to mannan from Saccharomyces cerevisiae induced an acute inflammation in inbred mouse strains resembling human Ps and PsA-like disease, whereas multiple injections induced a relapsing disease. Exacerbation of disease severity was observed in mice deficient for generation of reactive oxygen species (ROS). Interestingly, restoration of ROS production, specifically in macrophages, ameliorated both skin and joint disease. Neutralization of IL-17A, mainly produced by gammadelta T cells, completely blocked disease symptoms. Furthermore, mice depleted of granulocytes were resistant to disease development. In contrast, certain acute inflammatory mediators (C5, Fcgamma receptor III, mast cells, and histamine) and adaptive immune players (alphabeta T and B cells) were redundant in disease induction. Hence, we propose that mannan-induced activation of macrophages leads to TNF-alpha secretion and stimulation of local gammadelta T cells secreting IL-17A. The combined action of activated macrophages and IL-17A produced in situ drives neutrophil infiltration in the epidermis and dermis of the skin, leading to disease manifestations. Thus, our finding suggests a new mechanism triggered by exposure to exogenous microbial components, such as mannan, that can induce and exacerbate Ps and PsA.
in vivo IL-17A neutralization
Kulcsar, K. A., et al (2014). "Interleukin 10 modulation of pathogenic Th17 cells during fatal alphavirus encephalomyelitis" Proc Natl Acad Sci U S A 111(45): 16053-16058.
PubMed
Mosquito-borne alphaviruses are important causes of epidemic encephalomyelitis. Neuronal cell death during fatal alphavirus encephalomyelitis is immune-mediated; however, the types of cells involved and their regulation have not been determined. We show that the virus-induced inflammatory response was accompanied by production of the regulatory cytokine IL-10, and in the absence of IL-10, paralytic disease occurred earlier and mice died faster. To determine the reason for accelerated disease in the absence of IL-10, immune responses in the CNS of IL-10(-/-) and wild-type (WT) mice were compared. There were no differences in the amounts of brain inflammation or peak virus replication; however, IL-10(-/-) animals had accelerated and increased infiltration of CD4(+)IL-17A(+) and CD4(+)IL-17A(+)IFNgamma(+) cells compared with WT animals. Th17 cells infiltrating the brain demonstrated a pathogenic phenotype with the expression of the transcription factor, Tbet, and the production of granzyme B, IL-22, and GM-CSF, with greater production of GM-CSF in IL-10(-/-) mice. Therefore, in fatal alphavirus encephalomyelitis, pathogenic Th17 cells enter the CNS at the onset of neurologic disease and, in the absence of IL-10, appear earlier, develop into Th1/Th17 cells more often, and have greater production of GM-CSF. This study demonstrates a role for pathogenic Th17 cells in fatal viral encephalitis.
in vivo IL-17A neutralization
Uddin, M. N., et al (2014). "TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma" J Immunol 192(4): 1946-1953.
PubMed
Using several tumor models, we demonstrate that mice deficient in Bcl11b in T cells, although having reduced numbers of T cells in the peripheral lymphoid organs, developed significantly less tumors compared with wild-type mice. Bcl11b(-/-) CD4(+) T cells, with elevated TNF-alpha levels, but not the Bcl11b(-/-) CD8(+) T cells, were required for the reduced tumor burden, as were NK1.1(+) cells, found in increased numbers in Bcl11b(F/F)/CD4-Cre mice. Among NK1.1(+) cells, the NK cell population was predominant in number and was the only population displaying elevated granzyme B levels and increased degranulation, although not increased proliferation. Although the number of myeloid-derived suppressor cells was increased in the lungs with metastatic tumors of Bcl11b(F/F)/CD4-Cre mice, their arginase-1 levels were severely reduced. The increase in NK cell and myeloid-derived suppressor cell numbers was associated with increased bone marrow and splenic hematopoiesis. Finally, the reduced tumor burden, increased numbers of NK cells in the lung, and increased hematopoiesis in Bcl11b(F/F)/CD4-Cre mice were all dependent on TNF-alpha. Moreover, TNF-alpha treatment of wild-type mice also reduced the tumor burden and increased hematopoiesis and the numbers and activity of NK cells in the lung. In vitro treatment with TNF-alpha of lineage-negative hematopoietic progenitors increased NK and myeloid differentiation, further supporting a role of TNF-alpha in promoting hematopoiesis. These studies reveal a novel role for TNF-alpha in the antitumor immune response, specifically in stimulating hematopoiesis and increasing the numbers and activity of NK cells.
in vivo IL-17A neutralization
Xin, L., et al (2014). "Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2" Proc Natl Acad Sci U S A 111(29): 10672-10677.
PubMed
The costimulatory B7-1 (CD80)/B7-2 (CD86) molecules, along with T-cell receptor stimulation, together facilitate T-cell activation. This explains why in vivo B7 costimulation neutralization efficiently silences a variety of human autoimmune disorders. Paradoxically, however, B7 blockade also potently moderates accumulation of immune-suppressive regulatory T cells (Tregs) essential for protection against multiorgan systemic autoimmunity. Here we show that B7 deprivation in mice overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas peripherally induced Tregs retained in the absence of B7 selectively mitigate intestinal inflammation caused by Th17 effector CD4(+) T cells. The need for additional immune suppression in the intestine reflects commensal microbe-driven T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Eradication of commensal enteric bacteria mitigates intestinal inflammation and IL-17 production triggered by Treg depletion in B7-deficient mice, whereas re-establishing intestinal colonization with Candida albicans primes expansion of Th17 cells with commensal specificity. Thus, neutralizing B7 costimulation uncovers an essential role for Tregs in selectively averting intestinal inflammation by Th17 CD4(+) T cells with commensal microbe specificity.
in vivo IL-17A neutralization
Berger, H., et al (2013). "SOCS3 transactivation by PPARgamma prevents IL-17-driven cancer growth" Cancer Res 73(12): 3578-3590.
PubMed
Activation of the transcription factor PPARgamma by the n-3 fatty acid docosahexaenoic acid (DHA) is implicated in controlling proinflammatory cytokine secretion, but the intracellular signaling pathways engaged by PPARgamma are incompletely characterized. Here, we identify the adapter-encoding gene SOCS3 as a critical transcriptional target of PPARgamma. SOCS3 promoter binding and gene transactivation by PPARgamma was associated with a repression in differentiation of proinflammatory T-helper (TH)17 cells. Accordingly, TH17 cells induced in vitro displayed increased SOCS3 expression and diminished capacity to produce interleukin (IL)-17 following activation of PPARgamma by DHA. Furthermore, naive CD4 T cells derived from mice fed a DHA-enriched diet displayed less capability to differentiate into TH17 cells. In two different mouse models of cancer, DHA prevented tumor outgrowth and angiogenesis in an IL-17-dependent manner. Altogether, our results uncover a novel molecular pathway by which PPARgamma-induced SOCS3 expression prevents IL-17-mediated cancer growth.
in vivo IL-17A neutralization
Gladiator, A., et al (2013). "Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection" J Immunol 190(2): 521-525.
PubMed
IL-17-mediated immunity has emerged as a crucial host defense mechanism against fungal infections. Although Th cells are generally thought to act as the major source of IL-17 in response to Candida albicans, we show that fungal control is mediated by IL-17-secreting innate lymphoid cells (ILCs) and not by Th17 cells. By using a mouse model of oropharyngeal candidiasis we found that IL-17A and IL-17F, which are both crucial for pathogen clearance, are produced promptly upon infection in an IL-23-dependent manner, and that ILCs in the oral mucosa are the main source for these cytokines. Ab-mediated depletion of ILCs in RAG1-deficient mice or ILC deficiency in retinoic acid-related orphan receptor c(-/-) mice resulted in a complete failure to control the infection. Taken together, our data uncover the cellular basis for the IL-23/IL-17 axis, which acts right at the onset of infection when it is most needed for fungal control and host protection.
in vivo IL-17A neutralization
Gonzalez-Lombana, C., et al (2013). "IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection" PLoS Pathog 9(3): e1003243.
PubMed
Leishmaniasis, resulting from infection with the protozoan parasite Leishmania, consists of a wide spectrum of clinical manifestations, from healing cutaneous lesions to fatal visceral infections. A particularly severe form of cutaneous leishmaniasis, termed mucosal leishmaniasis, exhibits decreased IL-10 levels and an exaggerated inflammatory response that perpetuates the disease. Using a mouse model of leishmaniasis, we investigated what cytokines contribute to increased pathology when IL-10-mediated regulation is absent. Leishmania major infected C57BL/6 mice lacking IL-10 regulation developed larger lesions than controls, but fewer parasites. Both IFN-gamma and IL-17 levels were substantially elevated in mice lacking the capacity to respond to IL-10. IFN-gamma promoted an increased infiltration of monocytes, while IL-17 contributed to an increase in neutrophils. Surprisingly, however, we found that IFN-gamma did not contribute to increased pathology, but instead regulated the IL-17 response. Thus, blocking IFN-gamma led to a significant increase in IL-17, neutrophils and disease. Similarly, the production of IL-17 by cells from leishmaniasis patients was also regulated by IL-10 and IFN-gamma. Additional studies found that the IL-1 receptor was required for both the IL-17 response and increased pathology. Therefore, we propose that regulating IL-17, possibly by downregulating IL-1beta, may be a useful approach for controlling immunopathology in leishmaniasis.
in vivo IL-17A neutralization
Valdez, P. A., et al (2012). "Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells" Immunity 36(4): 668-679.
PubMed
T helper 17 (Th17) cells play an important role in mucosal host defense through production of the signature cytokines IL-17 and IL-22. Prostaglandin E2 (PGE2) has been shown to enhance IL-17 production by mature Th17 cells. However, when present during Th17 cell differentiation, we found that PGE2 inhibited the transcription factor IRF4 and suppressed production of IL-17 but not IL-22. We show that IRF4 was required for IL-17 expression but inhibited IL-22 expression, highlighting the potential for discordant regulation of these two cytokines in Th17 cells. The pathogenic fungus Cryptococcus neoformans produces PGE2, and we found that it uses PGE2- and IRF4-dependent mechanisms to specifically inhibit induction of IL-17 during Th17 cell differentiation. Blockade of host PGE2 during infection led to increased IL-17 production from CD4(+) T cells and increased survival of mice. These findings suggest that host- or pathogen-derived PGE2 can act directly on Th17 cells during differentiation to inhibit IL-17-dependent antimicrobial responses.
Product Citations
-
-
Immunology and Microbiology
Comparative analysis of Bacillus subtilis spores and monophosphoryl lipid A as adjuvants of protein-based mycobacterium tuberculosis-based vaccines: partial requirement for interleukin-17a for induction of protective immunity.
In Clinical and Vaccine Immunology : CVI on 1 April 2014 by Esparza-Gonzalez, S. C., Troy, A. R., et al.
PubMed
The development of adjuvants for vaccines has become an important area of research as the number of protein-based vaccines against infectious pathogens increases. Currently, there are a number of adjuvant-based Mycobacterium tuberculosis vaccines in clinical trials that have shown efficacy in animal models. Despite these novel adjuvants, there is still a need to design new and more versatile adjuvants that have minimal adverse side effects but produce robust long-lasting adaptive immune responses. To this end, we hypothesized that Bacillus subtilis spores may provide the appropriate innate signals that are required to generate such vaccine-mediated responses, which would be sufficient to reduce the mycobacterial burden after infection with M. tuberculosis. In addition, we compared the response generated by B. subtilis spores to that generated by monophosphoryl lipid A (MPL), which has been used extensively to test tuberculosis vaccines. The well-characterized, 6-kDa early secretory antigenic target of M. tuberculosis (ESAT-6; Rv3875) was used as a test antigen to determine the T cell activation potential of each adjuvant. Inoculated into mice, B. subtilis spores induced a strong proinflammatory response and Th1 immunity, similar to MPL; however, unlike MPL formulated with dimethyldioctadecylammonium (DDA) bromide, it failed to induce significant levels of interleukin-17A (IL-17A) and was unable to significantly reduce the mycobacterial burden after pulmonary infection with M. tuberculosis. Further analysis of the activity of MPL-DDA suggested that IL-17A was required for protective immunity. Taken together, the data emphasize the requirement for a network of cytokines that are essential for protective immunity.
-
-
-
Immunology and Microbiology
-
Cancer Research
IL-17-producing γδ T cells in the tumor microenvironment promote radioresistance in mice.
In J Clin Invest on 7 October 2025 by Deng, Y., Liu, X., et al.
PubMed
The immunosuppressive tumor microenvironment (TME) drives radioresistance, but the role of γδ T cells in regulating radiosensitivity remains incompletely understood. In this study, we found that γδ T cell infiltration in the TME substantially increased after radiotherapy and contributed to radioresistance. Depletion of γδ T cells enhanced radiosensitivity. Single-cell RNA sequencing revealed that γδ T cells in the post-radiotherapy TME were characterized by the expression of Zbtb16, Il23r, and Il17a, and served as the primary source of IL-17A. These γδ T cells promoted radioresistance by recruiting myeloid-derived suppressor cells and suppressing T cell activation. Mechanistically, radiotherapy-induced tumor cell-derived microparticles containing dsDNA activated the cGAS-STING/NF-κB signaling pathway in macrophages, upregulating the expression of the chemokine CCL20, which was critical for γδ T cell recruitment. Targeting γδ T cells and IL-17A enhanced radiosensitivity and improved the efficacy of radiotherapy combined with anti-PD-1 immunotherapy, providing potential therapeutic strategies to overcome radioresistance.
-
-
-
Immunology and Microbiology
-
Cardiovascular biology
Interleukin-17A-Related Inflammation Mediates Cardiac Injury in Chronic Relapsing Psoriasis-Like Mouse Model.
In Exp Dermatol on 1 July 2025 by Hu, M., Cao, H., et al.
PubMed
Psoriasis is an inflammatory disease characterised by chronic recurrent relapses. Previous observational studies have shown that patients with psoriasis are predisposed to cardiovascular comorbidities, but few studies have investigated the impact of psoriasis-related chronic inflammation on cardiac function. In this study, we used imiquimod (IMQ) to establish psoriasis-like mouse models with short-term inflammation (IMQ-ST) or long-term repeated inflammation (IMQ-LT), to mimic acute or chronic recurrent pathophysiology of psoriasis inflammation. The inflammatory pattern in the hearts of IMQ-ST mice and IMQ-LT mice was similar to that in the skin, characterised by increased level of interleukin (IL)-17A and proportion of IL-17A-producing γδT cells. However, only IMQ-LT mice showed declined cardiac function, significant myocardial tissue necrosis, and decreased expression of genes encoding structural and functional proteins in cardiomyocytes. Furthermore, IL-17A neutralisation markedly alleviated myocardial injury and improved cardiac function in IMQ-LT mice. In conclusion, we demonstrated that IL-17A-mediated inflammation was present in the skin and heart of acute and chronic psoriasis-like mouse models. However, only IMQ-LT mice developed myocardial injury and declined cardiac function, which could be prevented by IL-17A neutralisation.
-
-
The gut microbiome controls reactive astrocytosis during Aβ amyloidosis via propionate-mediated regulation of IL-17.
In J Clin Invest on 1 July 2025 by Chandra, S., Popović, J., et al.
PubMed
Accumulating evidence implicates the gut microbiome (GMB) in the pathogenesis and progression of Alzheimer's disease (AD). We recently showed that the GMB regulates reactive astrocytosis and Aβ plaque accumulation in a male APPPS1-21 AD mouse model. Yet, the mechanism(s) by which GMB perturbation alters reactive astrocytosis in a manner that reduces Aβ deposition remain unknown. Here, we performed metabolomics on plasma from mice treated with antibiotics (ABX) and identified a significant increase in plasma propionate, a gut-derived short-chain fatty acid, only in male mice. Administration of sodium propionate reduced reactive astrocytosis and Aβ plaques in APPPS1-21 mice, phenocopying the ABX-induced phenotype. Astrocyte-specific RNA-Seq on ABX- and propionate-treated mice showed reduced expression of proinflammatory and increased expression of neurotrophic genes. Next, we performed flow cytometry experiments, in which we found that ABX and propionate decreased peripheral RAR-related orphan receptor-γ+ (Rorγt+) CD4+ (Th17) cells and IL-17 secretion, which positively correlated with reactive astrocytosis. Last, using an IL-17 mAb to deplete IL-17, we found that propionate reduced reactive astrocytosis and Aβ plaques in an IL-17-dependent manner. Together, these results suggest that gut-derived propionate regulates reactive astrocytosis and Aβ amyloidosis by decreasing peripheral Th17 cells and IL-17 release. Thus, propionate treatment or strategies boosting propionate production may represent novel therapeutic strategies for the treatment of AD.
-
-
Cardiovascular biology
-
Immunology and Microbiology
Periodontitis aggravates pulmonary fibrosis by Porphyromonas gingivalis-promoted infiltration of neutrophils and Th17 cells.
In Front Cell Infect Microbiol on 11 June 2025 by Ye, H. L., Meng, X. Q., et al.
PubMed
Idiopathic pulmonary fibrosis (IPF) is a fatal interstitial lung disease. However, the pathogeny of IPF is poorly understood, and therapeutic options are very limited. Periodontitis (PD) is a chronic inflammatory disease that leads to dysbiosis of both the oral microbiome and host immune responses. While previous studies have suggested a PD-IPF association, insights into the mechanisms remain limited.
-
-
-
Immunology and Microbiology
BMAL1 and YAP Cooperate to hijack enhancers and promote inflammation in the Aged Epidermis
In Research Square on 8 May 2025 by Benitah, S., Bonjoch, J., et al.
-
-
-
Cardiovascular biology
-
Neuroscience
Low-Intensity Pulsed Ultrasound Promotes Oligodendrocyte Maturation and Remyelination by Down-regulating the Interleukin-17A/Notch1 Signaling Pathway in Mice with Ischemic Stroke.
In Research (Wash D C) on 28 April 2025 by Wang, J., Gao, Y., et al.
PubMed
Increasing evidence indicates that oligodendrocyte (OL) numbers and myelin as a dynamic cellular compartment perform a key role in the maintenance of neuronal function. Inhibiting white matter (WM) demyelination or promoting remyelination has garnered interest for its potential therapeutic strategy against ischemic stroke. Our previous work has shown that low-intensity pulsed ultrasound (LIPUS) could improve stroke recovery. However, it is unclear whether LIPUS can maintain WM integrity early after stroke or promote late WM repair. This study evaluated the efficacy of LIPUS on WM repair and long-term neurologic recovery after stroke. Male adult C57BL/6 mice underwent a focal cerebral ischemia model and were randomized to receive ultrasound stimulation (30 min once daily for 14 days). The effect of LIPUS on sensorimotor function was assessed by modified neurological severity score, rotarod test, grip strength test, and gait analysis up to 28 days after stroke. We found that ischemic stroke-induced WM damage was severe on day 7 and partially recovered on day 28. LIPUS prevented neuronal and oligodendrocyte progenitor cell (OPC) death during the acute phase of stroke (d7), protected WM integrity, and reduced brain atrophy and tissue damage during the recovery phase (d28). To further confirm the effect of LIPUS on remyelination, we assessed the proliferation and differentiation of OPCs. We found that LIPUS did not increase the number of OPCs (PDGFRα+ or NG2+), but markedly increased the number of newly produced mature OLs (APC+) and myelin protein levels. Mechanistically, LIPUS may promote OL maturation and remyelination by down-regulating the interleukin-17A/Notch1 signaling pathway. In summary, LIPUS can protect OLs and neurons early after stroke and promote long-term WM repair and functional recovery. LIPUS will be a viable strategy for the treatment of ischemic stroke in the future.
-
-
-
Immunology and Microbiology
BMAL1 and YAP cooperate to hijack enhancers and promote inflammation in the aged epidermis
In bioRxiv on 22 April 2025 by Bonjoch, J., Solá, P., et al.
-
-
-
Immunology and Microbiology
The Emerging Fungal Pathogen Candida auris Induces IFNγ to Colonize the Skin.
In PLoS Pathog on 1 April 2025 by Das, D., Ganesh, S. M., et al.
PubMed
Candida auris is an emerging multidrug-resistant skin-tropic fungal pathogen that causes serious human infections. However, the factors that regulate C. auris skin infection in vivo are still unclear. In this study, we identified that, unlike Candida albicans, which induces IL-17-secreting protective effector Th17 cells, C. auris predominately induces IFNγ-secreting pathogenic Th1 cells during reinfection. Surprisingly, we found that IFNγ enhances skin infection of C. auris but not C. albicans. Mechanistically, IFNγ enhances skin infection of C. auris by dampening the protective IL-17 responses and increasing dermal damage. Furthermore, we identified that the development of Th1 cells occurs through IL-12, produced by C. auris-induced inflammatory macrophages and monocyte-derived dendritic cells. In addition, our findings reveal that C. auris unique cell wall outer mannan layer regulates the development of Th1 and Th17 cells. Collectively, our findings, for the first time, identified that C. auris induces IFNγ to persist in the skin. These findings help explain why C. auris but not C. albicans preferentially persist in the skin long-term, with the potential to identify novel therapeutic approaches to prevent and treat this emerging fungal pathogen in humans.
-
-
-
Biochemistry and Molecular biology
-
Genetics
-
Neuroscience
IL-17A Induces Circadian Disruptions Through the Epigenetic Repression of BMAL1 in Mice With Alzheimer's Disease.
In J Cell Mol Med on 1 April 2025 by Liu, T., Mao, T., et al.
PubMed
Circadian disruptions and neuroinflammation impact nearly all people with Alzheimer's disease (AD), but their relationships with each other and the impact of their interaction on AD remain to be addressed. Here, we found that amyloid (A)-β treatment downregulated brain and muscle aryl hydrocarbon receptor nuclear translocator-like (BMAL) 1 through the hypermethylation of its promoter region in HT22 cells and that the inhibition of DNA methylation ameliorated circadian rhythm disorders and restored BMAL1 protein expression by reversing its hypermethylation in APPswe/PSEN1dE9 (APP/PS1) mice. Critically, increased levels of interleukin (IL)-17A contributed to BMAL1 downregulation through the hypermethylation of its promoter region, thus leading to circadian disruptions in APP/PS1 mice. Moreover, we revealed that the mitogen-activated protein kinase (MAPK) pathway was responsible for IL-17A-induced DNA methyltransferase (DNMT) 1 upregulation. Taken together, we elucidate a new mechanism connecting IL-17A with altered DNA methylation of Bmal1, which results in circadian disturbances in an AD mouse model.
-
-
-
Immunology and Microbiology
NETs-CD44-IL-17A Feedback Loop Drives Th17-Mediated Inflammation in Behçet's Uveitis.
In Adv Sci (Weinh) on 1 April 2025 by Wu, Y., Ning, K., et al.
PubMed
Behçet's uveitis (BU) is a severe ocular manifestation of Behçet's disease, typically accompanied by abnormal neutrophil infiltration and hyperactivation. However, the underlying causes of excessive neutrophil extracellular traps (NETs) production and mechanisms by which NETs contribute to the pathogenesis of BU remain incompletely understood. Neutrophils from BU patients exhibit a higher propensity for NETs release compared to healthy controls. In the experimental autoimmune uveitis (EAU), neutrophils are observed to exert pro-inflammatory effects through NETs. Clearing NETs can inhibit T helper 17 (Th17) cell differentiation and significantly alleviate EAU symptoms. In vivo and in vitro experiments demonstrate neutralizing IL-17A markedly reducing neutrophil infiltration and NETs formation in EAU. Single-cell RNA sequencing confirms that CD44 plays a key role in mediating interactions between NETs and Th17 cells. Antagonizing CD44 inhibits the proportion of Th17 cells and NETs formation. Multiplex immunofluorescence and cell communication analyses further demonstrate interactions and colocalization between NETs and CD44highCD4+T cells in EAU. NETs induce Th17 differentiation via upregulating CD44, and in turn, Th17 cells secrete IL-17A to recruit neutrophils and promote NETs formation. Interrupting NETs-CD44-IL-17A feedback loop may be a potential therapeutic target for BU.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Pathobiont-induced suppressive immune imprints thwart T cell vaccine responses.
In Nat Commun on 16 December 2024 by Hajam, I. A., Tsai, C. M., et al.
PubMed
Pathobionts have evolved many strategies to coexist with the host, but how immune evasion mechanisms contribute to the difficulty of developing vaccines against pathobionts is unclear. Meanwhile, Staphylococcus aureus (SA) has resisted human vaccine development to date. Here we show that prior SA exposure induces non-protective CD4+ T cell imprints, leading to the blunting of protective IsdB vaccine responses. Mechanistically, these SA-experienced CD4+ T cells express IL-10, which is further amplified by vaccination and impedes vaccine protection by binding with IL-10Rα on CD4+ T cell and inhibit IL-17A production. IL-10 also mediates cross-suppression of IsdB and sdrE multi-antigen vaccine. By contrast, the inefficiency of SA IsdB, IsdA and MntC vaccines can be overcome by co-treatment with adjuvants that promote IL-17A and IFN-γ responses. We thus propose that IL-10 secreting, SA-experienced CD4+ T cell imprints represent a staphylococcal immune escaping mechanism that needs to be taken into consideration for future vaccine development.
-
-
-
Cancer Research
-
Immunology and Microbiology
IL-17 signaling protects against Helicobacter pylori-induced gastric cancer.
In Gut Microbes on 26 November 2024 by Brackman, L. C., Jung, M. S., et al.
PubMed
Helicobacter pylori infection is the predominant risk factor for the development of gastric cancer. Risk is enhanced by specific H. pylori virulence factors, diet, and the inflammatory response. Chronic activation of T helper (Th) 1 and Th17 pathways contributes to prolonged inflammation; yet, higher expression of IL-17 receptor (IL-17RA) is a favorable prognostic marker for survival after gastric cancer diagnosis. The protective impact of IL-17RA signaling is not understood. To investigate if IL-17RA signaling protects during H. pylori-induced carcinogenesis, the transgenic InsGAStg/tg mouse, which is prone to H. pylori-induced gastric cancer, was utilized. InsGAStg/tg mice and InsGAStg/tgIl17ra-/- mice were infected with a cag type 4 secretion system (T4SS) positive H. pylori strain for up to 6 months. Six weeks post-infection, IL-17RA deficiency led to increased bacterial burden, increased gastritis, and development of lymphoid follicles. Increased inflammation was associated with heightened cellular proliferation and earlier loss of parietal and chief cells in InsGAStg/tgIl17ra-/- mice. Gastric cancers developed more frequently by 3- and 6-months post-infection in H. pylori-infected InsGAStg/tgIl17ra-/- mice compared to InsGAStg/tg mice. Chronic inflammation was exacerbated with IL-17RA deficiency, characterized by elevated Th1/Th17 cytokines, increased B cell infiltration, and enhanced IgA production, despite reduced expression of the polymeric immunoglobulin receptor. Further, paragastric lymph nodes of InsGAStg/tgIl17ra-/- mice were enlarged relative to controls and displayed altered gene expression profiles. Increased inflammation was accompanied by a significant increase in Cybb expression, which encodes NADPH oxidase 2, suggesting that increased oxidative damage may occur in the absence of IL-17RA. Further, there is increased phosphorylation of histone 2AX in IL-17RA deficient mice, indicating that the DNA damage response is highly activated. These data suggest that IL-17RA signaling activates a protective pathway to prevent excessive inflammation which otherwise can lead to increased oxidative stress, DNA damage, and drive gastric carcinogenesis after H. pylori infection.
-
-
-
Immunology and Microbiology
-
Neuroscience
MrgprA3 neurons drive cutaneous immunity against helminths through selective control of myeloid-derived IL-33.
In Nat Immunol on 1 November 2024 by Inclan-Rico, J. M., Napuri, C. M., et al.
PubMed
Skin uses interdependent cellular networks for barrier integrity and host immunity, but most underlying mechanisms remain obscure. Herein, we demonstrate that the human parasitic helminth Schistosoma mansoni inhibited pruritus evoked by itch-sensing afferents bearing the Mas-related G-protein-coupled receptor A3 (MrgprA3) in mice. MrgprA3 neurons controlled interleukin (IL)-17+ γδ T cell expansion, epidermal hyperplasia and host resistance against S. mansoni through shaping cytokine expression in cutaneous antigen-presenting cells. MrgprA3 neuron activation downregulated IL-33 but induced IL-1β and tumor necrosis factor in macrophages and type 2 conventional dendritic cells partially through the neuropeptide calcitonin gene-related peptide. Macrophages exposed to MrgprA3-derived secretions or bearing cell-intrinsic IL-33 deletion showed increased chromatin accessibility at multiple inflammatory cytokine loci, promoting IL-17/IL-23-dependent changes to the epidermis and anti-helminth resistance. This study reveals a previously unrecognized intercellular communication mechanism wherein itch-inducing MrgprA3 neurons initiate host immunity against skin-invasive parasites by directing cytokine expression patterns in myeloid antigen-presenting cell subsets.
-
-
-
Mus musculus (Mouse)
-
Cancer Research
Breast cancer colonization by Malassezia globosa accelerates tumor growth.
In MBio on 16 October 2024 by Liu, M. -. M., Zhu, H. -. H., et al.
PubMed
Malassezia globosa is a lipophilic basidiomycetous yeast that occurs abundantly in breast tumors and that may contribute to a shortened overall survival of breast cancer (BRAC) patients, suggesting that the yeast may participate in the carcinogenesis of BRAC. However, the mechanisms involved in the M. globosa-based acceleration of BRAC are unknown. Here, we show that M. globosa can colonize mammary tissue in 7,12-dimethylbenz[a] anthracene-induced mice. The abundance of M. globosa shortened the overall survival and increased the tumor incidence. Transcriptome data illustrated that IL-17A plays a key role in tumor growth due to M. globosa colonization, and tumor-associated macrophage infiltration was elevated during M. globosa colonization which triggers M2 polarization of macrophages via toll-like receptors 4/nuclear factor kappa-B (Nf-κB) signaling. Our results show that the expression of sphingosine kinase 1 (Sphk1) is increased in breast tumors after inoculation with M. globosa. Moreover, we discovered that Sphk1-specific small interfering RNA blocked the formation of lipid droplets, which can effectively alleviate the expression of the signal transducer and activator of the transcription 3 (STAT3)/Nf-κB pathway. Taken together, our results demonstrate that M. globosa could be a possible factor for the progression of BRAC. The mechanisms by which M. globosa promotes BRAC development involve the IL-17A/macrophage axis. Meanwhile, Sphk1 overexpression was induced by M. globosa infection, which also promoted the proliferation of MCF-7 cells.IMPORTANCELiterature has suggested that Malassezia globosa is associated with breast tumors; however, this association has not been confirmed. Here, we found that M. globosa colonizes in breast fat pads leading to tumor growth. As a lipophilic yeast, the expression of sphingosine kinase 1 (Sphk1) was upregulated to promote tumor growth after M. globosa colonization. Moreover, the IL-17A/macrophages axis plays a key role in mechanisms involved in the M. globosa-induced breast cancer acceleration from the tumor immune microenvironment perspective.
-
-
-
Mus musculus (Mouse)
-
Endocrinology and Physiology
-
Immunology and Microbiology
Pregnancy enhances antiviral immunity independent of type I IFN but dependent on IL-17-producing γδ+ T cells in the nasal mucosa.
In Sci Adv on 27 September 2024 by Chronopoulos, J., Pernet, E., et al.
PubMed
Pregnancy is associated with profound changes in immunity. However, pregnancy-related respiratory immune adaptations in response to influenza infection and their impact on disease severity remain unclear. Here, we describe, in a preclinical model of mid-gestation pregnancy, a mechanism of enhanced host defense against influenza A virus (IAV) localized to the nasal cavity that limits viral replication and reduces the magnitude of intrapulmonary immune responses. Consequently, the pregnant mice show reduced pulmonary pathology and preserved airway function after IAV infection. The early restriction of viral replication is independent of type I interferon (IFN) but dependent on increased antimicrobial peptides (AMPs) driven by interleukin-17+ (IL-17+) γδ+ T cells within the nasal passages. This pathway of host defense against IAV infection in the upper airways during pregnancy restricts early viral infection and prevents virus dissemination into the lung supporting maternal fitness.
-
-
-
Mus musculus (Mouse)
Hair follicles modulate skin barrier function.
In Cell Rep on 23 July 2024 by Ford, N. C., Benedeck, R. E., et al.
PubMed
Our skin provides a protective barrier that shields us from our environment. Barrier function is typically associated with the interfollicular epidermis; however, whether hair follicles influence this process remains unclear. Here, we utilize a potent genetic tool to probe barrier function by conditionally ablating a quintessential epidermal barrier gene, Abca12, which is mutated in the most severe skin barrier disease, harlequin ichthyosis. With this tool, we deduced 4 ways by which hair follicles modulate skin barrier function. First, the upper hair follicle (uHF) forms a functioning barrier. Second, barrier disruption in the uHF elicits non-cell-autonomous responses in the epidermis. Third, deleting Abca12 in the uHF impairs desquamation and blocks sebum release. Finally, barrier perturbation causes uHF cells to move into the epidermis. Neutralizing IL-17a, whose expression is enriched in the uHF, partially alleviated some disease phenotypes. Altogether, our findings implicate hair follicles as multi-faceted regulators of skin barrier function.
-
-
-
Mus musculus (Mouse)
-
Immunology and Microbiology
Clonal hematopoiesis driven by mutated DNMT3A promotes inflammatory bone loss.
In Cell on 11 July 2024 by Wang, H., Divaris, K., et al.
PubMed
Clonal hematopoiesis of indeterminate potential (CHIP) arises from aging-associated acquired mutations in hematopoietic progenitors, which display clonal expansion and produce phenotypically altered leukocytes. We associated CHIP-DNMT3A mutations with a higher prevalence of periodontitis and gingival inflammation among 4,946 community-dwelling adults. To model DNMT3A-driven CHIP, we used mice with the heterozygous loss-of-function mutation R878H, equivalent to the human hotspot mutation R882H. Partial transplantation with Dnmt3aR878H/+ bone marrow (BM) cells resulted in clonal expansion of mutant cells into both myeloid and lymphoid lineages and an elevated abundance of osteoclast precursors in the BM and osteoclastogenic macrophages in the periphery. DNMT3A-driven clonal hematopoiesis in recipient mice promoted naturally occurring periodontitis and aggravated experimentally induced periodontitis and arthritis, associated with enhanced osteoclastogenesis, IL-17-dependent inflammation and neutrophil responses, and impaired regulatory T cell immunosuppressive activity. DNMT3A-driven clonal hematopoiesis and, subsequently, periodontitis were suppressed by rapamycin treatment. DNMT3A-driven CHIP represents a treatable state of maladaptive hematopoiesis promoting inflammatory bone loss.
-
-
-
Immunology and Microbiology
Targeting STING in dendritic cells alleviates psoriatic inflammation by suppressing IL-17A production.
In Cell Mol Immunol on 1 July 2024 by Sun, X., Liu, L., et al.
PubMed
Psoriasis is a common chronic inflammatory skin disease driven by the aberrant activation of dendritic cells (DCs) and T cells, ultimately leading to increased production of cytokines such as interleukin (IL)-23 and IL-17A. It is established that the cGAS-STING pathway is essential for psoriatic inflammation, however, the specific role of cGAS-STING signaling in DCs within this context remains unclear. In this study, we demonstrated the upregulation of cGAS-STING signaling in psoriatic lesions by analyzing samples from both clinical patients and imiquimod (IMQ)-treated mice. Using a conditional Sting-knockout transgenic mouse model, we elucidated the impact of cGAS-STING signaling in DCs on the activation of IL-17- and IFN-γ-producing T cells in psoriatic inflammation. Ablation of the Sting hampers DC activation leads to decreased numbers of IL-17-producing T cells and Th1 cells, and thus subsequently attenuates psoriatic inflammation in the IMQ-induced mouse model. Furthermore, we explored the therapeutic potential of the STING inhibitor C-176, which reduces psoriatic inflammation and enhances the anti-IL-17A therapeutic response. Our results underscore the critical role of cGAS-STING signaling in DCs in driving psoriatic inflammation and highlight a promising psoriasis treatment.
-
-
-
Mus musculus (Mouse)
CXCL12+ dermal fibroblasts promote neutrophil recruitment and host defense by recognition of IL-17.
In J Exp Med on 1 April 2024 by Cavagnero, K. J., Li, F., et al.
PubMed
The skin provides an essential barrier for host defense through rapid action of multiple resident and recruited cell types, but the complex communication network governing these processes is incompletely understood. To define these cell-cell interactions more clearly, we performed an unbiased network analysis of mouse skin during invasive S. aureus infection and revealed a dominant role for CXCL12+ fibroblast subsets in neutrophil communication. These subsets predominantly reside in the reticular dermis, express adipocyte lineage markers, detect IL-17 and TNFα, and promote robust neutrophil recruitment through NFKBIZ-dependent release of CXCR2 ligands and CXCL12. Targeted deletion of Il17ra in mouse fibroblasts resulted in greatly reduced neutrophil recruitment and increased infection by S. aureus. Analogous human CXCL12+ fibroblast subsets abundantly express neutrophil chemotactic factors in psoriatic skin that are subsequently decreased upon therapeutic targeting of IL-17. These findings show that CXCL12+ dermal immune acting fibroblast subsets play a critical role in cutaneous neutrophil recruitment and host defense.
-