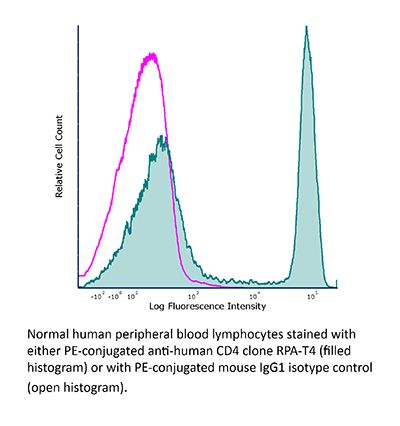
FlowMAb PE anti-human CD4
Product Description
Specifications
| Isotype | Mouse IgG1, κ |
|---|---|
| Recommended Isotype Control(s) | FlowMAb PE mouse IgG1 isotype control, unknown specificity |
| Conjugation | PE |
| Excitation Source | Yellow-Green 488 nm, 532 nm, 561 nm |
| Excitation Max | 496 nm, 566 nm |
| Emission Max | 576 nm |
| Immunogen | Not available or unknown |
| Reported Applications |
Immunofluorescence Immunohistochemistry (frozen) Flow cytometry |
| Protocol Information | It is recommended that the reagent be carefully titrated for optimal performance in the assay of interest. |
| Concentration | 0.2 mg/ml |
| Formulation |
PBS, pH 7.0 Contains 0.09% Sodium Azide |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G. Conjugated with R-phycoerythrin under optimal conditions. |
| Storage | The antibody solution should be stored at the stock concentration at 4°C and protected from prolonged exposure to light. Do not freeze. |
| Need a Custom Formulation? | See All Antibody Customization Options |
Application References
Immunofluorescence
Moolla, N., et al. (2016). "Thioredoxin (Trx1) regulates CD4 membrane domain localization and is required for efficient CD4-dependent HIV-1 entry" Biochim Biophys Acta 1860(9): 1854-1863.
PubMed
BACKGROUND: CD4 is a glycoprotein expressed on the surfaces of certain immune cells. On lymphocytes, an important function of CD4 is to co-engage Major Histocompatibility Complex (MHC) molecules with the T Cell Receptor (TCR), a process that is essential for antigen-specific activation of T cells. CD4 localizes dynamically into distinct membrane microdomains, an important feature of its immunoregulatory function that has also been shown to influence the efficiency of HIV replication. However, the mechanism by which CD4 localization is regulated and the biological significance of this is incompletely understood. METHODS: In this study, we used confocal microscopy, density-gradient centrifugation and flow cytometry to analyze dynamic redox-dependent effects on CD4 membrane domain localization. RESULTS: Blocking cell surface redox exchanges with both a membrane-impermeable sulfhydryl blocker (DTNB) and specific antibody inhibitors of Thioredoxin-1 (Trx1) induces translocation of CD4 into detergent-resistant membrane domains (DRM). In contrast, Trx1 inactivation does not change the localization of the chemokine receptor CCR5, suggesting that this effect is targeted. Moreover, DTNB treatment and Trx1 depletion coincide with strong inhibition of CD4-dependent HIV entry, but only moderate reductions in the infectivity of a CD4-independent HIV pseudovirion. CONCLUSIONS: Changes in the extracellular redox environment, potentially mediated by allosteric consequences of functional disulfide bond oxidoreduction, may represent a signal for translocation of CD4 into DRM clusters, and this sequestration, another potential mechanism by which the anti-HIV effects of cell surface oxidoreductase inhibition are exerted. GENERAL SIGNIFICANCE: Extracellular redox conditions may regulate CD4 function by potentiating changes in its membrane domain localization.
Immunohistochemistry (frozen)
Mack, C. L., et al. (2004). "Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation" Pediatr Res 56(1): 79-87.
PubMed
A proposed mechanism in the pathogenesis of biliary atresia involves an initial virus-induced, progressive T cell-mediated inflammatory obliteration of bile ducts. The aim of this study was to characterize the inflammatory environment present within the liver of infants with biliary atresia to gain insight into the role of a primary immune-mediated process versus a nonspecific secondary response to biliary obstruction. Frozen liver tissue obtained from patients with biliary atresia, neonatal giant cell hepatitis, total parenteral nutrition (TPN)-related cholestasis, choledochal cysts, and normal control subjects was used for fluorescent immunohistochemistry studies of cellular infiltrates, cytokine mRNA expression, and in situ hybridization for localization of cytokine-producing cells. Immunohistochemistry revealed increases in CD8(+) and CD4(+) T cells and Kupffer cells (CD68(+)) in the portal tracts of biliary atresia. Reverse transcription-PCR analysis of biliary atresia tissue showed a Th1-type cytokine profile with expression of IL-2, interferon-gamma, tumor necrosis factor-alpha, and IL-12. This profile was not seen in normal, neonatal hepatitis or choledochal cyst livers but was present in TPN-related cholestasis. In situ hybridization revealed that the Th1 cytokine-producing cells were located in the portal tracts in biliary atresia and in the parenchyma of TPN-related cholestasis. A distinctive portal tract inflammatory environment is present in biliary atresia, involving CD4(+) Th1 cell-mediated immunity. The absence of similar inflammation in other pediatric cholestatic conditions suggests that the portal tract inflammation in biliary atresia is not a secondary response to cholestasis but rather indicates a specific immune response involved in the pathogenesis of biliary atresia.
Flow Cytometry
Geijtenbeek, T. B., et al. (2000). "DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells" Cell 100(5): 587-597.
PubMed
Dendritic cells (DC) capture microorganisms that enter peripheral mucosal tissues and then migrate to secondary lymphoid organs, where they present these in antigenic form to resting T cells and thus initiate adaptive immune responses. Here, we describe the properties of a DC-specific C-type lectin, DC-SIGN, that is highly expressed on DC present in mucosal tissues and binds to the HIV-1 envelope glycoprotein gp120. DC-SIGN does not function as a receptor for viral entry into DC but instead promotes efficient infection in trans of cells that express CD4 and chemokine receptors. We propose that DC-SIGN efficiently captures HIV-1 in the periphery and facilitates its transport to secondary lymphoid organs rich in T cells, to enhance infection in trans of these target cells.