Antibody Structure
Introduction
First described in 1890, antibodies, also known as an immunoglobulins (Ig), are large Y-shaped glycoproteins that are used by the immune system to identify and neutralize foreign antigens, such as bacteria or viruses. Antibodies are able to bind virtually any non-self surface with exquisite specificity and high affinity. These attributes make antibodies not only key to immunity but also indispensable tools for biomedical research, diagnostics and therapy.
Antibody Structure
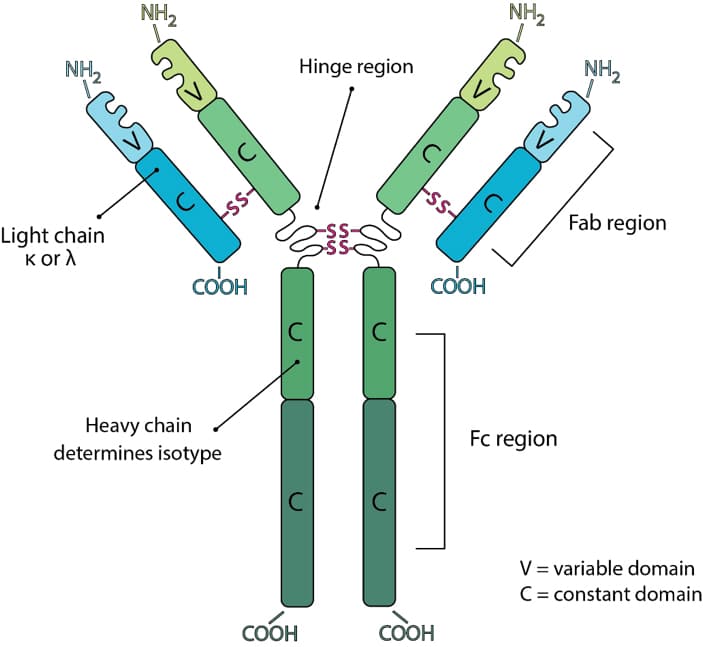
Antibodies consist of two heavy and two light chains. The light chain is classified as either a kappa (κ) or lambda (λ) chain based on small differences in the polypeptide sequence. The heavy chain defines the class or isotype of an antibody. Each component chain contains one NH2-terminal variable domain and one or more COOH-terminal constant domains. Each variable or constant domain consists of approximately 110–130 amino acids. Both light chains contain only one constant domain, whereas the heavy chains contain either three or four constant domains, which define the isotype. Heavy chains with three constant domains tend to include a spacer hinge region between the first and second constant domains. A typical light chain will have a mass of approximately 25 kDa and a three constant domain heavy chain with its hinge will have a mass of approximately 55 kDa.
Isotype
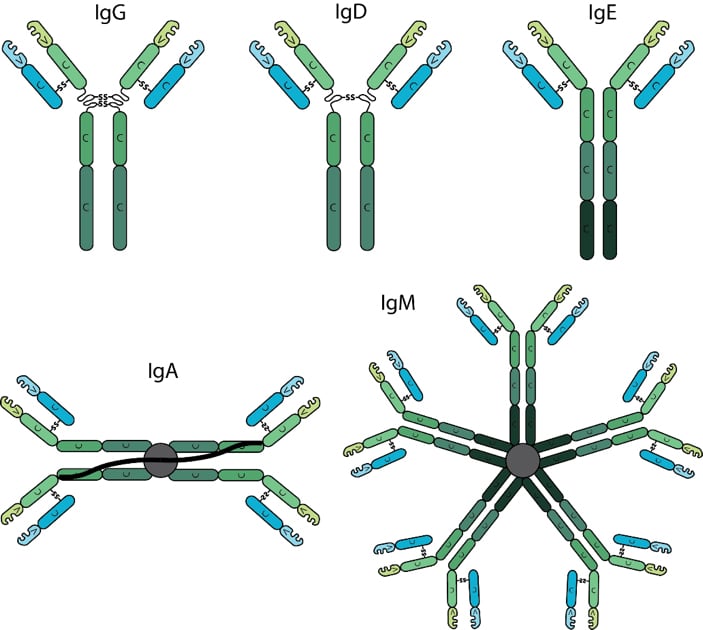
Antibodies are classified into isotypes which are defined by individual types of heavy chain constant domains, each isotype being encoded by a separate constant domain gene. Differences in the heavy chain constant domain between isotypes include the number and location of interchain disulfide bonds, the number of attached oligosaccharide moieties, the number of constant domains, and the length of the hinge region. In mammals, antibodies are divided into five isotypes: IgG, IgM, IgA, IgD and IgE. In humans and mice, the Immunoglobulin IgG is further divided into subclasses (e.g. in mouse IgG1, IgG2a, IgG2b, IgG2c and IgG3) based on small differences in the number of disulfide bonds and the length and flexibility of the hinge region. The various antibody isotypes differ in their biological features, structure, target specificity and distribution.
IgG
The most predominant isotype found in the body is IgG. It has the longest serum half-life of all immunoglobulin isotypes. IgG is further subdivided into four subclasses based on structural, antigenic and functional differences in the heavy chain constant region. The IgG subclasses exhibit different functional activities. For example, IgG1 and IgG3 antibodies are generally induced in response to protein antigens whereas IgG2 and IgG4 are associated with polysaccharide antigens.
IgD
Circulating IgD is found at very low levels in serum and has a short serum half-life. The function of IgD is unknown.
IgE
IgE is present at the lowest serum levels and has the lowest half-life of all antibody isotypes. IgE is associated with hypersensitivity and allergic reactions as well as the response to parasitic worm infections.
IgA
IgA serum levels tend to be higher than IgM, but considerably lower than IgG. IgA generally exists as a monomer in serum however, it can form a dimer where the third constant regions are bound to a polypeptide chain, the J-chain via disulfide bonds. IgA also contains a secretory component. The secretory component is disulfide bonded to the second constant domains of the dimer. IgA is critical at protecting against toxins, virus and bacteria by direct neutralization or by prevention of binding to mucosal surfaces.
IgM
The first antibody produced during an immune response is IgM. Upon maturation and antigenic stimulation, IgM units’ link to each other by disulfide bonds in the fourth constant region to form pentameric IgM. Pentameric IgM also contains the J-chain, which is bound to two of the monomers by a disulfide bond. Generally, monomeric IgM molecules have low affinity due to their immaturity however, pentameric IgM can achieve high avidity by means of multimeric interactions between the pentameric antibody and the antigen, especially if the antigen contains multiple repeating epitopes.
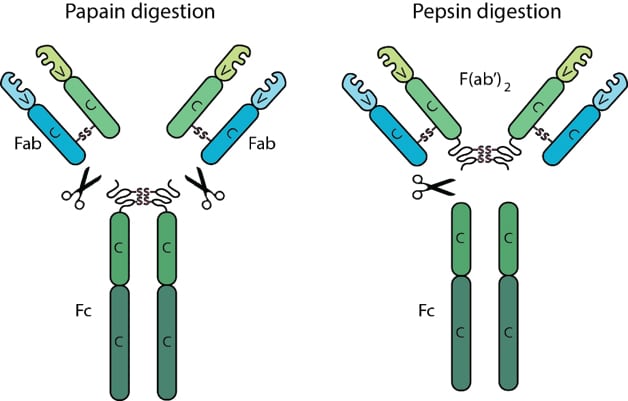
F(ab) and Fc Fragments
All antibodies contain two F(ab) regions and one Fc region. The F(ab) regions correspond to the two identical arms of the antibody molecule, which contain the complete light chains paired with the variable and first constant domains of the heavy chains. The Fc region corresponds to the constant domains of the heavy chain, excluding the first constant domain. These three regions, often referred to as fragments, can be separated via proteolytic enzyme digestion. Digestion with the enzyme papain cleaves antibodies into three fragments, two F(ab) fragments and one Fc fragment, while the enzyme pepsin does not separate the two F(ab) fragments resulting in one Fc fragment and one F(ab’)2 fragment consisting of both F(ab) fragments joined by disulfide bonds. The F(ab) and F(ab’)2 fragments retain the ability to bind antigen even after digestion making them ideal for certain immunochemical techniques and experimental applications.