InVivoMAb anti-mouse IL-12 p40
Product Details
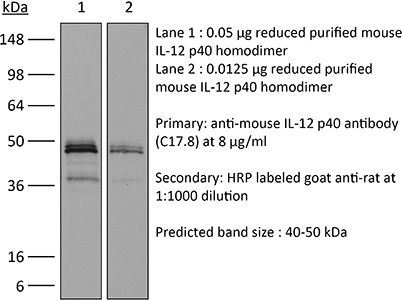
The C17.8 antibody reacts with mouse p40 also known as IL-12β. p40 is a 40 kDa subunit of IL-12 and IL-23. IL-12 is a heterodimeric cytokine composed of subunits IL-12α p35 and IL-12β p40. The p40 subunit of IL-12 also combines with p19, a protein that shows no biological activity by itself, to form IL-23. IL-12 is secreted by activated monocytes, macrophages, and dendritic cells while IL-23 is secreted by activated dendritic cells and epithelial cells. IL-12 plays roles in T lymphocyte differentiation, IFNγ production, and NK cell cytotoxicity. The C17.8 antibody has been shown to neutralize both IL-12 and IL-23 bioactivity.Specifications
| Isotype | Rat IgG2a, κ |
|---|---|
| Recommended Isotype Control(s) | InVivoMAb rat IgG2a isotype control, anti-trinitrophenol |
| Recommended Dilution Buffer | InVivoPure pH 7.0 Dilution Buffer |
| Conjugation | This product is unconjugated. Conjugation is available via our Antibody Conjugation Services. |
| Immunogen | Recombinant mouse IL-12 p70 |
| Reported Applications |
in vivo IL-12p40 neutralization p40 affinity chromatography Immunoprecipitation ELISA Flow cytometry Western blot |
| Formulation |
PBS, pH 7.0 Contains no stabilizers or preservatives |
| Endotoxin |
<2EU/mg (<0.002EU/μg) Determined by LAL gel clotting assay |
| Purity |
>95% Determined by SDS-PAGE |
| Sterility | 0.2 µm filtration |
| Production | Purified from cell culture supernatant in an animal-free facility |
| Purification | Protein G |
| RRID | AB_1107698 |
| Molecular Weight | 150 kDa |
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
Additional Formats
Recommended Products
in vivo IL-12 neutralization
Dann, S. M., et al. (2018). "Giardia Infection of the Small Intestine Induces Chronic Colitis in Genetically Susceptible Hosts" J Immunol 201(2): 548-559. PubMed
Chemokines are small chemotactic proteins that have a crucial role in leukocyte recruitment into tissue. Targeting these mediators has been suggested as a potential therapeutic option in inflammatory skin diseases such as psoriasis. Using quantitative RT-PCR, we found CCL7, a chemokine ligand known to interact with multiple C-C chemokine receptors, to be markedly increased in lesional psoriasis as opposed to atopic dermatitis, lichen planus, non-lesional psoriatic and normal control skin. Surprisingly, this increase in CCL7 mRNA expression exceeded that of all other chemokines investigated, and keratinocytes and dermal blood endothelial cells were identified as its likely cellular sources. In an imiquimod-induced psoriasis-like mouse model, CCL7 had a profound impact on myeloid cell inflammation as well as on the upregulation of key pro-psoriatic cytokines such as CCL20, IL-12p40 and IL-17C, while its blockade led to an increase in the antipsoriatic cytokine IL-4. In humans receiving the TNF-alpha-blocker infliximab, CCL7 was downregulated in lesional psoriatic skin already within 16 hours after a single intravenous infusion. These data suggest that CCL7 acts as a driver of TNF-alpha-dependent Th1/Th17-mediated inflammation in lesional psoriatic skin.
in vivo IL-12 neutralization, Flow Cytometry
Deligne, C., et al. (2015). "Anti-CD20 therapy induces a memory Th1 response through the IFN-gamma/IL-12 axis and prevents protumor regulatory T-cell expansion in mice" Leukemia 29(4): 947-957. PubMed
The long-lasting clinical response by lymphoma patients to anti-CD20 therapy has been attributed to the induction of an anti-tumor adaptive immunity. We previously demonstrated that a CD4-dependent mechanism is responsible for the long-term protection of CD20(+) tumor-bearing mice by anti-CD20 treatment. Here, we compare tumor immunity in tumor-bearing animals that did or did not receive anti-CD20 treatment. Splenic CD4(+)FoxP3(+) regulatory T cells (Tregs) expanded substantially in untreated mice that exhibited then a reduced survival, whereas Tregs depletion led to long-term survival of the animals, suggesting the establishment of a Treg-dependent immunosuppressive environment after tumor injection. Strikingly, anti-CD20 therapy reversed the initial expansion of Tregs, and was accompanied by a marked increase in the number of Th1 cells, with no detectable change in Th2 and Th17 cell numbers. Interleukin-12 serum level was also increased by the anti-CD20 treatment, and activated myeloid dendritic cells producing interleukin-12 could be detected in lymph nodes of treated animals, while interferon-gamma blockade strongly reduced survival. Also, CD4(+) effector memory T cells were evidenced in surviving animals, and the transfer of CD4(+) T cells induced long-term protection. Thus, anti-CD20 therapy promotes strong anti-tumor adaptive immunity, opposes Treg expansion and inhibits tumor cells from maintaining an immunosuppressive environment.
in vivo IL-12 neutralization
Brunner, P. M., et al. (2015). "CCL7 contributes to the TNF-alpha-dependent inflammation of lesional psoriatic skin" Exp Dermatol 24(7): 522-528. PubMed
Chemokines are small chemotactic proteins that have a crucial role in leukocyte recruitment into tissue. Targeting these mediators has been suggested as a potential therapeutic option in inflammatory skin diseases such as psoriasis. Using quantitative RT-PCR, we found CCL7, a chemokine ligand known to interact with multiple C-C chemokine receptors, to be markedly increased in lesional psoriasis as opposed to atopic dermatitis, lichen planus, non-lesional psoriatic and normal control skin. Surprisingly, this increase in CCL7 mRNA expression exceeded that of all other chemokines investigated, and keratinocytes and dermal blood endothelial cells were identified as its likely cellular sources. In an imiquimod-induced psoriasis-like mouse model, CCL7 had a profound impact on myeloid cell inflammation as well as on the upregulation of key pro-psoriatic cytokines such as CCL20, IL-12p40 and IL-17C, while its blockade led to an increase in the antipsoriatic cytokine IL-4. In humans receiving the TNF-alpha-blocker infliximab, CCL7 was downregulated in lesional psoriatic skin already within 16 hours after a single intravenous infusion. These data suggest that CCL7 acts as a driver of TNF-alpha-dependent Th1/Th17-mediated inflammation in lesional psoriatic skin.
in vivo IL-12 neutralization
Villegas-Mendez, A., et al. (2015). "Parasite-specific CD4+IFN-gamma+IL-10+ T cells distribute within both lymphoid and non-lymphoid compartments and are controlled systemically by IL-27 and ICOS during blood-stage malaria infection" Infect Immun. pii : IAI.01100-15. PubMed
Immune-mediated pathology in IL-10 deficient mice during blood-stage malaria infection typically manifests in non-lymphoid organs, such as the liver and lung. Thus, it is critical to define the cellular sources of IL-10 in these sensitive non-lymphoid compartments during infection. Moreover, it is important to determine if IL-10 production is controlled through conserved or disparate molecular programmes in distinct anatomical locations during malaria infection, as this may enable spatiotemporal tuning of the regulatory immune response. In this study, using dual IFN-gamma-YFP and IL-10-GFP reporter mice we show that CD4+YFP+ T cells are the major source of IL-10 in both lymphoid and non-lymphoid compartments throughout the course of blood-stage P. yoelii infection. Mature splenic CD4+YFP+GFP+ T cells, which preferentially expressed high levels of CCR5, were capable of migrating to and seeding the non-lymphoid tissues, indicating that the systemically distributed host-protective cells have a common developmental history. Despite exhibiting comparable phenotypes, CD4+YFP+GFP+ T cells from the liver and lung produced significantly higher quantities of IL-10 than their splenic counterparts, showing that the CD4+YFP+GFP+ T cells exert graded functions in distinct tissue locations during infection. Unexpectedly, given the unique environmental conditions within discrete non-lymphoid and lymphoid organs, we show that IL-10 production by CD4+YFP+ T cells is controlled systemically during malaria infection through IL-27R signalling that is supported post-CD4+ T cell priming by ICOS signalling. The results in this study substantially improve our understanding of the systemic IL-10 response to malaria infection, particularly within sensitive non-lymphoid organs.
Immunoprecipitation, p40 affinity chromatography
Abdi, K., et al. (2014). "Free IL-12p40 monomer is a polyfunctional adaptor for generating novel IL-12-like heterodimers extracellularly" J Immunol 192(12): 6028-6036. PubMed
IL-12p40 partners with the p35 and p19 polypeptides to generate the heterodimeric cytokines IL-12 and IL-23, respectively. These cytokines play critical and distinct roles in host defense. The assembly of these heterodimers is thought to take place within the cell, resulting in the secretion of fully functional cytokines. Although the p40 subunit alone can also be rapidly secreted in response to inflammatory signals, its biological significance remains unclear. In this article, we show that the secreted p40 monomer can generate de novo IL-12-like activities by combining extracellularly with p35 released from other cells. Surprisingly, an unbiased proteomic analysis reveals multiple such extracellular binding partners for p40 in the serum of mice after an endotoxin challenge. We biochemically validate the binding of one of these novel partners, the CD5 Ag-like glycoprotein, to the p40 monomer. Nevertheless, the assembled p40-CD5L heterodimer does not recapitulate the biological activity of IL-12. These findings underscore the plasticity of secreted free p40 monomer, suggesting that p40 functions as an adaptor that is able to generate multiple de novo composites in combination with other locally available polypeptide partners after secretion.
in vivo IL-12p40 neutralization
Ruffell, B., et al. (2014). "Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells" Cancer Cell 26(5): 623-637. PubMed
Blockade of colony-stimulating factor-1 (CSF-1) limits macrophage infiltration and improves response of mammary carcinomas to chemotherapy. Herein we identify interleukin (IL)-10 expression by macrophages as the critical mediator of this phenotype. Infiltrating macrophages were the primary source of IL-10 within tumors, and therapeutic blockade of IL-10 receptor (IL-10R) was equivalent to CSF-1 neutralization in enhancing primary tumor response to paclitaxel and carboplatin. Improved response to chemotherapy was CD8(+) T cell-dependent, but IL-10 did not directly suppress CD8(+) T cells or alter macrophage polarization. Instead, IL-10R blockade increased intratumoral dendritic cell expression of IL-12, which was necessary for improved outcomes. In human breast cancer, expression of IL12A and cytotoxic effector molecules were predictive of pathological complete response rates to paclitaxel.
in vivo IL-12p40 neutralization
Tarrio, M. L., et al. (2014). "Proliferation conditions promote intrinsic changes in NK cells for an IL-10 response" J Immunol 193(1): 354-363. PubMed
Constitutively found at high frequencies, the role for NK cell proliferation remains unclear. In this study, a shift in NK cell function from predominantly producing IFN-gamma, a cytokine with proinflammatory and antimicrobial functions, to producing the immunoregulatory cytokine IL-10 was defined during extended murine CMV infection. The response occurred at times subsequent to IL-12 production, but the NK cells elicited acquired responsiveness to IL-12 and IL-21 for IL-10 production. Because neither IL-12 nor IL-21 was required in vivo, however, additional pathways appeared to be available to promote NK cell IL-10 expression. In vitro studies with IL-2 to support proliferation and in vivo adoptive transfers into murine CMV-infected mice demonstrated that NK cell proliferation and further division enhanced the change. In contrast to the sustained open profile of the IFN-gamma gene, NK cells responding to infection acquired histone modifications in the IL-10 gene indicative of changing from a closed to an open state. The IL-10 response to IL-12 was proliferation dependent ex vivo if the NK cells had not yet expanded in vivo but independent if they had. Thus, a novel role for proliferation in supporting changing innate cell function is reported.
in vivo IL-12p40 neutralization
Tang, W., et al. (2014). "The oncoprotein and transcriptional regulator Bcl-3 governs plasticity and pathogenicity of autoimmune T cells" Immunity 41(4): 555-566. PubMed
Bcl-3 is an atypical member of the IkappaB family that modulates transcription in the nucleus via association with p50 (NF-kappaB1) or p52 (NF-kappaB2) homodimers. Despite evidence attesting to the overall physiologic importance of Bcl-3, little is known about its cell-specific functions or mechanisms. Here we demonstrate a T-cell-intrinsic function of Bcl-3 in autoimmunity. Bcl-3-deficient T cells failed to induce disease in T cell transfer-induced colitis and experimental autoimmune encephalomyelitis. The protection against disease correlated with a decrease in Th1 cells that produced the cytokines IFN-gamma and GM-CSF and an increase in Th17 cells. Although differentiation into Th1 cells was not impaired in the absence of Bcl-3, differentiated Th1 cells converted to less-pathogenic Th17-like cells, in part via mechanisms involving expression of the RORgammat transcription factor. Thus, Bcl-3 constrained Th1 cell plasticity and promoted pathogenicity by blocking conversion to Th17-like cells, revealing a unique type of regulation that shapes adaptive immunity.
in vivo IL-12p40 neutralization
Yu, X., et al. (2013). "A multifunctional chimeric chaperone serves as a novel immune modulator inducing therapeutic antitumor immunity" Cancer Res 73(7): 2093-2103. PubMed
Converting the immunosuppressive tumor environment into one that is favorable to the induction of antitumor immunity is indispensable for effective cancer immunotherapy. Here, we strategically incorporate a pathogen (i.e., flagellin)-derived, NF-kappaB-stimulating “danger” signal into the large stress protein or chaperone Grp170 (HYOU1/ORP150) that was previously shown to facilitate antigen crosspresentation. This engineered chimeric molecule (i.e., Flagrp170) is capable of transporting tumor antigens and concurrently inducing functional activation of dendritic cells (DC). Intratumoral administration of adenoviruses expressing Flagrp170 induces a superior antitumor response against B16 melanoma and its distant lung metastasis compared with unmodified Grp170 and flagellin. The enhanced tumor destruction is accompanied with significantly increased tumor infiltration by CD8(+) cells as well as elevation of IFN-gamma and interleukin (IL)-12 levels in the tumor sites. In situ Ad.Flagrp170 therapy provokes systemic activation of CTLs that recognize several antigens naturally expressing in melanoma (e.g., gp100/PMEL and TRP2/DCT). The mechanistic studies using CD11c-DTR transgenic mice and Batf3-deficient mice reveal that CD8alpha(+) DCs are required for the improved T-cell crosspriming. Antibody neutralization assays show that IL-12 and IFN-gamma are essential for the Flagrp170-elicited antitumor response, which also involves CD8(+) T cells and natural killer cells. The therapeutic efficacy of Flagrp170 and its immunostimulating activity are also confirmed in mouse prostate cancer and colon carcinoma. Together, targeting the tumor microenvironment with this chimeric chaperone is highly effective in mobilizing or restoring antitumor immunity, supporting the potential therapeutic use of this novel immunomodulator in the treatment of metastatic diseases.
in vivo IL-12p40 neutralization
Gwyer Findlay, E., et al. (2013). "IL-27 receptor signaling regulates CD4+ T cell chemotactic responses during infection" J Immunol 190(9): 4553-4561. PubMed
IL-27 exerts pleiotropic suppressive effects on naive and effector T cell populations during infection and inflammation. Surprisingly, however, the role of IL-27 in restricting or shaping effector CD4(+) T cell chemotactic responses, as a mechanism to reduce T cell-dependent tissue inflammation, is unknown. In this study, using Plasmodium berghei NK65 as a model of a systemic, proinflammatory infection, we demonstrate that IL-27R signaling represses chemotaxis of infection-derived splenic CD4(+) T cells in response to the CCR5 ligands, CCL4 and CCL5. Consistent with these observations, CCR5 was expressed on significantly higher frequencies of splenic CD4(+) T cells from malaria-infected, IL-27R-deficient (WSX-1(-/-)) mice than from infected wild-type mice. We find that IL-27 signaling suppresses splenic CD4(+) T cell CCR5-dependent chemotactic responses during infection by restricting CCR5 expression on CD4(+) T cell subtypes, including Th1 cells, and also by controlling the overall composition of the CD4(+) T cell compartment. Diminution of the Th1 response in infected WSX-1(-/-) mice in vivo by neutralization of IL-12p40 attenuated CCR5 expression by infection-derived CD4(+) T cells and also reduced splenic CD4(+) T cell chemotaxis toward CCL4 and CCL5. These data reveal a previously unappreciated role for IL-27 in modulating CD4(+) T cell chemotactic pathways during infection, which is related to its capacity to repress Th1 effector cell development. Thus, IL-27 appears to be a key cytokine that limits the CCR5-CCL4/CCL5 axis during inflammatory settings.
in vivo IL-12p40 neutralization
Villegas-Mendez, A., et al. (2013). "IL-27 receptor signalling restricts the formation of pathogenic, terminally differentiated Th1 cells during malaria infection by repressing IL-12 dependent signals" PLoS Pathog 9(4): e1003293. PubMed
The IL-27R, WSX-1, is required to limit IFN-gamma production by effector CD4(+) T cells in a number of different inflammatory conditions but the molecular basis of WSX-1-mediated regulation of Th1 responses in vivo during infection has not been investigated in detail. In this study we demonstrate that WSX-1 signalling suppresses the development of pathogenic, terminally differentiated (KLRG-1(+)) Th1 cells during malaria infection and establishes a restrictive threshold to constrain the emergent Th1 response. Importantly, we show that WSX-1 regulates cell-intrinsic responsiveness to IL-12 and IL-2, but the fate of the effector CD4(+) T cell pool during malaria infection is controlled primarily through IL-12 dependent signals. Finally, we show that WSX-1 regulates Th1 cell terminal differentiation during malaria infection through IL-10 and Foxp3 independent mechanisms; the kinetics and magnitude of the Th1 response, and the degree of Th1 cell terminal differentiation, were comparable in WT, IL-10R1(-)/(-) and IL-10(-)/(-) mice and the numbers and phenotype of Foxp3(+) cells were largely unaltered in WSX-1(-)/(-) mice during infection. As expected, depletion of Foxp3(+) cells did not enhance Th1 cell polarisation or terminal differentiation during malaria infection. Our results significantly expand our understanding of how IL-27 regulates Th1 responses in vivo during inflammatory conditions and establishes WSX-1 as a critical and non-redundant regulator of the emergent Th1 effector response during malaria infection.
in vivo IL-12p40 neutralization
Chappert, P., et al. (2013). "Specific gut commensal flora locally alters T cell tuning to endogenous ligands" Immunity 38(6): 1198-1210. PubMed
Differences in gut commensal flora can dramatically influence autoimmune responses, but the mechanisms behind this are still unclear. We report, in a Th1-cell-driven murine model of autoimmune arthritis, that specific gut commensals, such as segmented filamentous bacteria, have the ability to modulate the activation threshold of self-reactive T cells. In the local microenvironment of gut-associated lymphoid tissues, inflammatory cytokines elicited by the commensal flora dynamically enhanced the antigen responsiveness of T cells that were otherwise tuned down to a systemic self-antigen. Together with subtle differences in early lineage differentiation, this ultimately led to an enhanced recruitment of pathogenic Th1 cells and the development of a more severe form of autoimmune arthritis. These findings define a key role for the gut commensal flora in sustaining ongoing autoimmune responses through the local fine tuning of T-cell-receptor-proximal activation events in autoreactive T cells.
in vivo IL-12p40 neutralization
Prabhakara, R., et al. (2011). "Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus" Infect Immun 79(12): 5010-5018. PubMed
Staphylococcus aureus is a common cause of prosthetic implant infections, which can become chronic due to the ability of S. aureus to grow as a biofilm. Little is known about adaptive immune responses to these infections in vivo. We hypothesized that S. aureus elicits inflammatory Th1/Th17 responses, associated with biofilm formation, instead of protective Th2/Treg responses. We used an adapted mouse model of biofilm-mediated prosthetic implant infection to determine chronic infection rates, Treg cell frequencies, and local cytokine levels in Th1-biased C57BL/6 and Th2-biased BALB/c mice. All C57BL/6 mice developed chronic S. aureus implant infection at all time points tested. However, over 75% of BALB/c mice spontaneously cleared the infection without adjunctive therapy and demonstrated higher levels of Th2 cytokines and anti-inflammatory Treg cells. When chronic infection rates in mice deficient in the Th2 cytokine interleukin-4 (IL-4) via STAT6 mutation in a BALB/c background were assessed, the mice were unable to clear the S. aureus implant infection. Additionally, BALB/c mice depleted of Treg cells via an anti-CD25 monoclonal antibody (MAb) were also unable to clear the infection. In contrast, the C57BL/6 mice that were susceptible to infection were able to eliminate S. aureus biofilm populations on infected intramedullary pins once the Th1 and Th17 responses were diminished by MAb treatment with anti-IL-12 p40. Together, these results indicate that Th2/Treg responses are mechanisms of protection against chronic S. aureus implant infection, as opposed to Th1/Th17 responses, which may play a role in the development of chronic infection.
in vivo IL-12p40 neutralization
Mack, E. A., et al. (2011). "Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection" MBio 2(4). PubMed
Natural killer (NK) cells are equipped to innately produce the cytokine gamma interferon (IFN-gamma) in part because they basally express high levels of the signal transducer and activator of transcription 4 (STAT4). Type 1 interferons (IFNs) have the potential to activate STAT4 and promote IFN-gamma expression, but concurrent induction of elevated STAT1 negatively regulates access to the pathway. As a consequence, it has been difficult to detect type 1 IFN stimulation of NK cell IFN-gamma during viral infections in the presence of STAT1 and to understand the evolutionary advantage for maintaining the pathway. The studies reported here evaluated NK cell responses following infections with lymphocytic choriomeningitis virus (LCMV) in the compartment handling the earliest events after infection, the peritoneal cavity. The production of type 1 IFNs, both IFN-alpha and IFN-beta, was shown to be early and of short duration, peaking at 30 h after challenge. NK cell IFN-gamma expression was detected with overlapping kinetics and required activating signals delivered through type 1 IFN receptors and STAT4. It took place under conditions of high STAT4 levels but preceded elevated STAT1 expression in NK cells. The IFN-gamma response reduced viral burdens. Interestingly, increases in STAT1 were delayed in NK cells compared to other peritoneal exudate cell (PEC) populations. Taken together, the studies demonstrate a novel mechanism for stimulating IFN-gamma production and elucidate a biological role for type 1 IFN access to STAT4 in NK cells.
in vivo IL-12p40 neutralization, ELISA
Massacand, J. C., et al. (2009). "Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function" Proc Natl Acad Sci U S A 106(33): 13968-13973. PubMed
Thymic stromal lymphopoietin (TSLP) is an interleukin (IL)-7-like cytokine, mainly expressed by epithelial cells, and key to the development of allergic responses. The well-documented involvement of TSLP in allergy has led to the conviction that TSLP promotes the development of inflammatory Th2 cell responses. However, we now report that the interaction of TSLP with its receptor (TSLPR) has no functional impact on the development of protective Th2 immune responses after infection with 2 helminth pathogens, Heligmosomoides polygyrus and Nippostrongylus brasiliensis. Mice deficient in the TSLP binding chain of the TSLPR (TSLPR(-/-)) exhibited normal Th2 cell differentiation, protective immunity and memory responses against these two distinct rodent helminths. In contrast TSLP was found to be necessary for the development of protective Th2 responses upon infection with the helminth Trichuris muris (T. muris). TSLP inhibited IL-12p40 production in response to T. muris infection, and treatment of TSLPR(-/-) animals with neutralizing anti-IL-12p40 monoclonal antibody (mAb) was able to reverse susceptibility and attenuate IFN-gamma production. We additionally demonstrated that excretory-secretory (ES) products from H. polygyrus and N. brasiliensis, but not T. muris, were capable of directly suppressing dendritic cell (DC) production of IL-12p40, thus bypassing the need for TSLP. Taken together, our data show that the primary function of TSLP is to directly suppress IL-12 secretion, thus supporting Th2 immune responses.